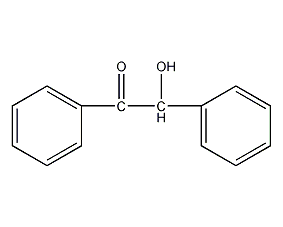
Structural formula
| Business number | 03B0 |
|---|---|
| Molecular formula | C14H12O2 |
| Molecular weight | 212.25 |
| label |
α-Hydroxy-α-phenylacetophenone, α-Hydroxybenzyl phenyl ketone, 2-Hydroxy-2-phenylacetophenone, C6H5CH(OH)COC6H5, disinfectant, catalyst, photoinitiator, Environmental catalyst |
Numbering system
CAS number:119-53-9
MDL number:MFCD00004496
EINECS number:204-331-3
RTECS number:DI1590000
BRN number:391839
PubChem ID:None
Physical property data
1. Properties: White crystal. Can reduce Fehling’s solution.
2. Density (g/mL, 25/4℃): 1.310
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 137
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 102.9kPa): 334
p>
7. Refractive index: Undetermined
8. Flash point (ºC): 181
9. Crystalline phase Standard heat of combustion (enthalpy) (kJ·mol -1): -6976.4
10. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): -247.7
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/ mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water ( Log value of the partition coefficient (octanol/water): Undetermined
17.Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: soluble in pyridine, boiling Ethanol and acetone, slightly soluble in ether. Soluble in 3335 parts of cold water and slightly more in hot water.
Toxicological data
1. Acute toxicity: Rat oral LD50: 1mg/kg
Mouse oral LD50: >3g/kg
Rabbit transdermal LD50: >8870mg/kg
Ecological data
Mildly hazardous to water.
Molecular structure data
1. Molar refractive index: 62.30
2. Molar volume (cm3/mol): 179.9
3. Isotonic specific volume (90.2K): 479.9
4. Surface tension (dyne/cm): 50.6
5. Polarizability (10-24cm3): 24.70
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 2
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 225
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidizing agents.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and water. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment.
Synthesis method
It is produced by the condensation of two molecules of benzaldehyde under the catalytic action of potassium cyanide (or sodium).
Purpose
Fluorescence reaction tests zinc. Standards for calorimetry. preservative. Organic Synthesis.

 微信扫一扫打赏
微信扫一扫打赏

