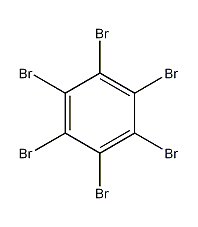
Structural formula
| Business number | 01YK |
|---|---|
| Molecular formula | C6Br6 |
| Molecular weight | 551.49 |
| label |
Perbrominated benzene, HBB, Added flame retardants, filler |
Numbering system
CAS number:87-82-1
MDL number:MFCD00000058
EINECS number:201-773-9
RTECS number:DA2200100
BRN number:1912586
PubChem number:24869043
Physical property data
1. Properties: white needle-like crystals
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL , air=1): Undetermined
4. Melting point (ºC): 320~326 (lit.)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2 kPa): Undetermined
7. Refractive index: Undetermined
p>
8. Flash point (ºC): -18
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC ): Undetermined
11. Vapor pressure (kPa, 25 ºC): Undetermined
12. Saturated vapor pressure (kPa, 60 ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
p>
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper limit of explosion (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: soluble in benzene, slightly soluble in ethanol and ether, insoluble in water
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 72.39
2. Molar volume (cm3/mol): 186.5
3. Isotonic specific volume (90.2K ): 510.2
4. Surface tension (dyne/cm): 55.9
5. Polarizability (10-24cm3): 28.69
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 104
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters:0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14. Uncertain chemical bond stereocenters Number of centers: 0
15. Number of covalent bond units: 1
Properties and stability
Insoluble in water, high hydrolytic stability
Storage method
This product should be sealed and stored in a cool, dry place. Store in a cool and dry place.
Synthesis method
1. Preparation method using fuming sulfuric acid as solvent: In a kettle-type stirred reactor, first put a certain amount of fuming sulfuric acid, then add benzene, stir and dissolve, then add a small amount of iron triiodide (or iodine and iron powder) as the catalyst, control the reaction temperature to about 80°C, add 6 parts of bromine dropwise, and finish the addition within 9 hours. Then heat to increase the temperature. When the temperature reaches 150°C, add 6 parts of bromine dropwise while stirring. After the addition, continue stirring for 1 hour, the reaction will be complete, and water-insoluble hexabromobenzene will be generated and suspended in the reaction material. After cooling, filtering, washing and drying, the finished product is obtained, with a yield of about 72% to 73%. The hydrogen bromide generated during the reaction is extracted and absorbed with water as a by-product. The product wash liquid is neutralized with alkali. The filtration mother liquor is mainly solvent sulfuric acid and can be recycled.
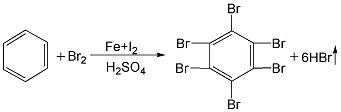
2. Preparation method using tetrachloroethane as solvent: First put tetrachloroethane and benzene into the reaction kettle, stir and mix, and then add an appropriate amount of iodine and iron powder as catalyst. Control the temperature at 80°C and add bromine dropwise while stirring. Keep the reaction temperature at 80°C and react for 10 hours. After the reaction is completed, the reactants are cooled, crystallized, filtered, washed, and dried to obtain the finished product. The filtered mother liquor is mainly tetrachloroethane, which is recycled and used. Wash water needs to be treated with alkali. During the reaction, hydrogen bromide is vacuumed out and water is absorbed as a by-product, hydrobromic acid. The yield of this method is about 92%.
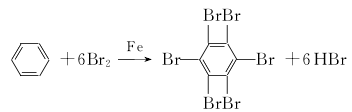
3.It is equipped with a stirrer, condenser (the upper port is connected to the gas receiving bottle filled with water through a pipe), thermometer and dripping liquid Add 1.5g of iron powder into the 500mL four-necked flask of the funnel, then inject 476g of bromine (2.9784mol), control the temperature at 40°C, and stir for 15 minutes to allow the iron and bromine to fully react. Then lower the temperature to about 8°C, control the temperature to 8-15°C, and add 16g (0.2045mol) benzene dropwise in 1 hour while stirring. Then slowly raise the temperature, control it for 30 to 40 minutes to raise the temperature to 60°C, and stir at this temperature for 2.5 hours to complete the bromination reaction (the HBr gas escaped during the bromination process is absorbed into the gas receiving bottle, and then treated with Cl2 Recover bromine), when no gas is discharged and a large amount of solid appears in the bottle, let it cool to room temperature naturally. Remove the four-neck bottle, add 150 mL of water, change to a distillation device, and steam out unreacted bromine. When the temperature rises to 100°C, heating is stopped and the solid in the bottle turns white. When cooled to 60°C, add 150 to 200 mL of the above product hydrochloric acid into the four-neck bottle, then boil for 1 hour, and try to break up the agglomerates so that the solid can be fully mixed with the hydrochloric acid. After stopping heating, cool to room temperature, and then Carry out suction filtration, wash the filter cake with water until neutral, and dry it by controlling the temperature at 100-120°C to obtain 110-112g of white crystalline hexabromobenzene, with a melting point of 320-326°C and a yield of more than 95%. The halogenation reaction formula is as follows
4.Add 150mL bromine into a 500mL four-neck round bottom flask equipped with a stirrer, thermometer, constant pressure dropping funnel, spherical condenser tube and tail gas absorption device and 2g of anhydrous aluminum trichloride, cool to below 10°C and start adding 8g of benzene dropwise, and finish dropping in about 1 hour. During the dripping process, the temperature is controlled below 10°C. After the dripping is completed, slowly heat up to reflux (about 50 minutes), reflux for 4.5 hours, cool, add an appropriate amount of water, evaporate excess bromine, and maintain rapid stirring during the steamer process to make the product highly dispersed. Distillation can be stopped when the gas phase temperature reaches about 100°C, and a yellow solid can be obtained by filtration. Wash the crude product with dilute hydrochloric acid until the color becomes lighter, then add 100 mL of mixed solvent and stir at room temperature for 0.5 to 1 hour. After filtration, a white solid is obtained. The dry weight was 55.59g, the yield was 98%, the melting point was 324-325°C, and the purity measured by HPLC was 99.6%.
Purpose
This product is an additive flame retardant, suitable for polyethylene, polypropylene, polystyrene, ABS resin, epoxy resin, polyamide, polyester, etc. It has a synergistic effect when used together with antimony trioxide at (2~3):1. This product has good flame resistance and self-extinguishing effects, and has little impact on the physical properties of the resin. This product has excellent heat resistance and can be effectively used for plastics that require high-temperature molding. It has good compatibility with resin, high melting point, and smooth appearance of molded products. This product can also be used as filler.

 微信扫一扫打赏
微信扫一扫打赏

