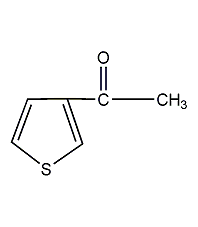
Structural formula
| Business number | 01Z3 |
|---|---|
| Molecular formula | C6H6OS |
| Molecular weight | 126.18 |
| label |
2-thiophene ethanone, Thiophene-2-ethanone, Methyl-2-thienyl ketone, 1-(2-Thienyl)ethanone, Multifunctional solvents, Extracting agent |
Numbering system
CAS number:88-15-3
MDL number:MFCD00005442
EINECS number:201-804-6
RTECS number:OB6300000
BRN number:107910
PubChem number:24845180
Physical property data
1. Properties: colorless liquid.
2. Density (g/mL, 20/4℃): 1.1679
3. Melting point (ºC): 10-11
4. Boiling point ( ºC, normal pressure): 213.5
5. Boiling point (ºC, 1.73kpa): 94-96
6. Refractive index (20ºC): 1.5667
7. Flash point (ºC): 91
8. Solubility: Easily soluble in ether and miscible with organic solvents.
Toxicological data
Harmful if inhaled, taken orally or in contact with skin.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 34.66
2. Molar volume (cm3/mol): 110.4
3. Isotonic specific volume (90.2K ): 275.6
4. Surface tension (dyne/cm): 38.7
5. Polarizability (10-24cm3): 13.74
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecule polar surface area 45.3
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 101
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Storage method
This product should be sealed and stored in a cool, dry place away from light.
Synthesis method
Thiophene reacts with acetic anhydride in the presence of phosphoric acid. Put acetic anhydride, 85% phosphoric acid and thiophene into the reaction pot, stir, gradually heat to reflux temperature (96-97°C) and maintain reflux for 2.5 hours, cool, wash with water, and separate the water layer. The obtained oil layer is first subjected to normal pressure and then reducedDistill under pressure, collect the distillate, let it stand, separate the water layer, and recover the thiophene for reuse in the next batch of materials. The oil remaining in the pot is distilled under reduced pressure, and the 89-90°C (1.33kPa) fraction is collected to obtain the finished product. The yield is 85.7%. A similar method involves the reaction of thiophene with acetyl chloride in the presence of tin tetrachloride.
Purpose
Used as solvent, extractant, and pharmaceutical intermediate.

 微信扫一扫打赏
微信扫一扫打赏

