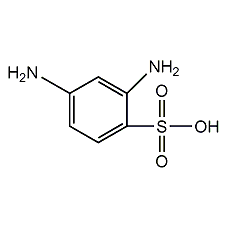
Structural formula
| Business number | 01ZS |
|---|---|
| Molecular formula | C6H8N2O3S |
| Molecular weight | 188.20 |
| label |
m-diaminobenzene sulfonic acid, 2,4-diaminobenzenesulfonic acid, 2,4-Diaminobenzenesulfonic Acid, aromatic sulfur compounds |
Numbering system
CAS number:88-63-1
MDL number:MFCD00035772
EINECS number:201-846-5
RTECS number:DB5900000
BRN number:2694057
PubChem number:24859688
Physical property data
1. Properties: light brown crystals, the crystals precipitated from water are monoclinic crystals.
2. Solubility: Easily soluble in hot water, slightly soluble in cold water, and gradually turns brown in the air.
Toxicological data
Highly toxic.
Ecological data
None
Molecular structure data
1. Molar refractive index: 44.33
2. Molar volume (cm3/mol): 116.7
3. Isotonic specific volume (90.2K ): 356.1
4. Surface tension (dyne/cm): 86.5
5. Polarizability (10-24cm3): 17.57
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 115
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 246
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Inhalation of its vapor or absorption through the skin can cause poisoning. The workplace should be well ventilated and operators should wear protective equipment.
Storage method
For its packaging, storage and transportation, please refer to m-aminobenzene sulfonic acid.
Synthesis method
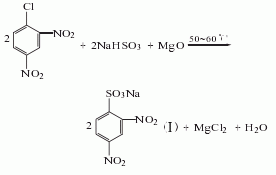
Using 2,4-dinitrochlorobenzene as raw material, perform a sulfonation reaction with sodium bisulfite in the presence of magnesium oxide. After cooling, add hydrochloric acid to It becomes acidic, and 2,4-dinitrobenzene sulfonic acid is obtained by adding salt, cooling, letting it stand, suction filtration, and centrifugal dehydration. Then put it into a reduction pot filled with iron filings and hydrochloric acid, boil it, neutralize it with Na2CO3, then add NaHSO3, let it stand, and filter it. The filtrate is concentrated, crystallized, and centrifuged to obtain the finished product
![]()
Purpose
Dye intermediates. Mainly used to manufacture acid dyes, neutral dyes and various reactive dyes, such as acid medium brown RH, reactive bright yellow K-5G, reactive brilliant blue K-GR, neutral brilliant yellow S-5GL and other dyes.

 微信扫一扫打赏
微信扫一扫打赏

