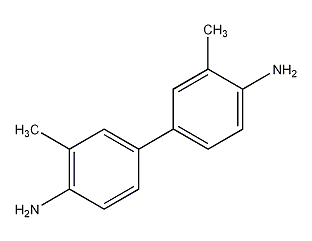
Structural formula
| Business number | 03BH |
|---|---|
| Molecular formula | C14H16N12 |
| Molecular weight | 212.29 |
| label |
o-Toluidine, o-Toluidine; diaminodimethylbiphenyl, o-Tolidine, 4-4’Diamino-3-3′-dimethylbiphenyl, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:119-93-7
MDL number:MFCD00014773
EINECS number:204-358-0
RTECS number:DD1225000
BRN number:2210640
PubChem number:24900054
Physical property data
1. Properties: White to reddish flaky crystals. [1]
2. Melting point (℃): 129~131[2]
3. Boiling point (℃) :300~339[3]
4. Relative density (water=1): 1.0[4]
5 .Octanol/water partition coefficient: 2.34[5]
6. Solubility: insoluble in water, soluble in ethanol, ether, and acetic acid. [6]
Toxicological data
1. Acute toxicity[7] LD50: 404mg/kg (rat oral)
2. Irritation No data yet
3. Mutagenicity [8] Microbial mutagenicity: Salmonella typhimurium 100μg/dish. Sister chromatid exchange: rabbit lymphocytes 50mg/L. Unprogrammed DNA synthesis: human HeLa cells 1 μmol/L. DNA damage: human lymphocytes 500 μmol/L (2h)
4. Carcinogenicity [9] IARC Carcinogenicity Comment: G2B, suspected carcinogenic to humans things.
Ecological data
1. Ecotoxicity No data yet
2. Biodegradability[10]
Aerobic biodegradation (h): 24~168
Anaerobic biodegradation (h): 96~672
3. Non-biodegradability[11]
Photooxidation half-life in water (h): 31.2~1740
Photooxidation half-life in air (h): 0.267~2.67
4. Bioaccumulation [12] BCF: 5~10 (carp, contact concentration 0.02mg/L, contact time 8 weeks); 34~83 (carp, exposure concentration 0.2mg/L, exposure time 8 weeks)
5. Other harmful effects [13] This substance has The environment is hazardous and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 68.96
2. Molar volume (cm3/mol): 191.8
3. Isotonic specific volume (90.2K ): 507.5
4. Surface tension (dyne/cm): 49.0
5. Polarizability (10-24cm3): 27.34
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:��
6. Topological molecule polar surface area 52
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 205
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain atomic stereocenter Number of stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Total Number of price key units: 1
Properties and stability
1. Stability[14] Stable
2. Incompatible substances[15] Strong oxidants, strong acids
3. Conditions to avoid contact[16] Heating
4 .Hazards of aggregation[17] No aggregation
Storage method
Storage Precautions[18] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Use zinc powder (or ferrosilicon powder) to reduce o-nitrotoluene to 2,2′-dimethyl symmetric diphenylhydrazine in caustic soda medium, and then add 22.5% sulfuric acid and sodium metabisulfite (or In the presence of hydrochloric acid), rearrange to form 3,3′-dimethylbenzidine sulfate (or hydrochloride), add 30% liquid caustic soda, and deacidify to obtain the finished product.
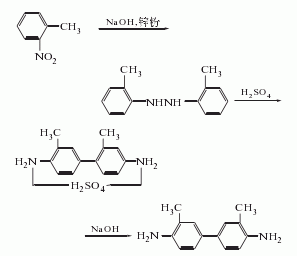
2. The preparation method uses industrial product di-o-toluidine as raw material and purifies the reagent di-o-toluidine. Add 15kg of industrial hydrochloric acid with a relative density of 1.60 to 200L of distilled water, heat to 50-60°C, slowly add 12kg of industrial di-o-toluidine while stirring, add activated carbon and metastannic acid in sequence after complete dissolution, stir and filter, and add metastantin to the filtrate. Filter the stannic acid once, and slowly add 5% ammonia water at 50 to 60°C to the filtrate obtained with sufficient stirring until the pH value of the solution is 8 to 9. Filter while it is hot. Wash the obtained crystals with distilled water until the pH of the washing liquid = 7, and then use Dissolve in distilled water, add an aqueous solution of EDTA acid adjusted to a pH value of 5 to 6 with ammonia, stir evenly and leave it for 24 hours (continuous stirring during this period), then filter, wash once with distilled water, and filter dry to get the finished product.
Purpose
1. Photometric determination of gold, cerium and free chlorine. Determination of nitrate, manganese, nitrite, chromium salts, copper salts, cobalt, mercury and cyanide ions in the air.
2. Used as a chromogen for qualitative detection or photometric determination of gold. It is also used for the detection of free chlorine, nitrates and cyanide particles in water. Nitrite, chromium salts, copper salts, cobalt, mercury and air.
3. Dye intermediates. Mainly used to produce naphthol AS-G, cyan base R, direct blue 3B, direct dark green 2B-NB, direct dark brown B-NM, direct yellow-brown ND3G, direct black BN, direct red G, direct lake blue 5B, direct Copper salt blue 2R and various organic pigments, etc. It can also be used as a measuring agent for cobalt, copper, chromium and other metals, a reagent for measuring moisture and a redox indicator.
4. Used as a cross-linking agent for dyes, ulai sugar resin, and a reagent for identifying gold and free chlorine in water. [19]

 微信扫一扫打赏
微信扫一扫打赏

