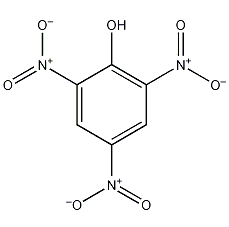
Structural formula
| Business number | 0206 |
|---|---|
| Molecular formula | C6H3N3O7 |
| Molecular weight | 229 |
| label |
Chloropicrin, Picric acid chloride, picric acid, Picric acid |
Numbering system
CAS number:88-89-1
MDL number:MFCD00007102
EINECS number:201-865-9
RTECS number:TJ7875000
BRN number:423400
PubChem number:24854392
Physical property data
1. Properties: light yellow crystalline solid, odorless, bitter taste. [1]
2. Melting point (℃): 122~123[2]
3. Boiling point (℃) : 300 (explosion) [3]
4. Relative density (water = 1): 1.763[4]
5. Relative vapor density (air=1): 7.90[5]
6. Saturated vapor pressure (kPa): <0.133 (20℃ )[6]
7. Heat of combustion (kJ/mol): -2558.0[7]
8. Alcohol/water partition coefficient: 1.33~2.03[8]
9. Flash point (℃): 150 (CC) [9]
10. Ignition temperature (℃): 300 (explosion) [10]
11. Solubility: soluble in water, ethanol, benzene, ether, Acetone, pyridine, etc. [11]
Toxicological data
1. Acute toxicity[12] LD50: 200mg/kg (rat oral); 56.3mg/kg (mouse intraperitoneal)
2. Irritation No data available
3. Mutagenicity[13] Microbial mutagenicity: rats Salmonella typhi 5μmol/dish; Escherichia coli 1800ppm. Sex chromosome deletion and non-disjunction: Drosophila melanogaster orally administered 1250 μmol/L.
Ecological data
1. Ecotoxicity[14] LC50: 109mg/L (96h) (rainbow trout); 170mg/L (96h) (bluegill sunfish)
2. Biodegradability [15] MITI-I test, initial concentration 100mg/L, sludge concentration 30mg/L, degradation after 4 weeks twenty three%.
3. Non-biodegradability No information available
4. Other harmful effects[16] This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 47.77
2. Molar volume (cm3/mol): 123.3
3. Isotonic specific volume (90.2K ): 388.7
4. Surface tension (dyne/cm): 98.5
5. Polarizability (10-24cm3): 18.93
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 7
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. Topological molecule polar surface area 158
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 292
10. Isotopic atomsQuantity: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine Number of stereocenters of chemical bonds: 0
14. Number of stereocenters of uncertain chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[17] Stable
2. Incompatible substances[18] Strong oxidant, strong alkali, heavy metal powder
3. Conditions to avoid contact[19] Heat, friction, vibration, impact
4. Polymerization hazard[20] No polymerization
5. Decomposition products[21] Nitrogen oxides
Storage method
Storage Precautions[22] Store in a cool, dry and ventilated warehouse dedicated to explosives. Keep away from fire and heat sources. The storage temperature does not exceed 32°C and the relative humidity does not exceed 80%. If it contains water as a stabilizer, the storage temperature should not be lower than 1℃ and the relative humidity should be less than 80%. They should be stored separately from oxidants, alkalis, and heavy metal powders, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. Suitable materials should be available in the storage area to contain spills. Vibration, impact and friction are prohibited.
Synthesis method
(1) Synthesis of sodium 2,4-dinitrophenolate The mixture of 2,4-dinitrochlorobenzene and 2,6-dinitrochlorobenzene is hydrolyzed with sodium hydroxide at 100°C. A mixture of 2,4-dinitrophenolate sodium and 2,6-dinitrophenolate sodium was prepared.
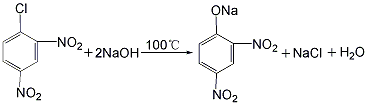
(2 ) Synthesis of 2,4-dinitrophenol: The sodium 2,4-dinitrophenol prepared above is acidified with sulfuric acid to obtain 2,4-dinitrophenol.
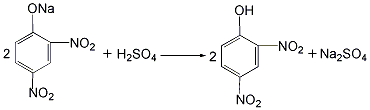
(3) 2,4, Synthesis of 6-trinitrophenol: The 2,4-dinitrophenol prepared above is nitrated with a mixed acid of concentrated sulfuric acid and nitric acid at 60 to 65°C. The product is filtered by suction and washed with water to obtain 2,4,6-trinitrophenol.
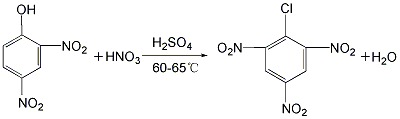
4. Change 2,4- The mixture of dinitrochlorobenzene and 2,6-dinitrochlorobenzene is hydrolyzed with sodium hydroxide to obtain a mixture of 2,4-dinitrophenolate sodium and 2,6-dinitrophenolate sodium. After acidification with sulfuric acid, it is nitrated with a mixed acid of concentrated sulfuric acid and nitric acid. The product is filtered by suction and washed with water to obtain sodium dinitrophenolate.
Purpose
1. Colorimetric analysis to determine potassium. Gravimetric determination of bismuth. Test for cyanide. Organic analysis determines aromatic hydrocarbons, amines, phenols, alkaloids, aromatic esters and heterocyclic compounds. Fixative for microscopy. Explosive materials. tanning. Battery. Etched copper. Manufacturing of stained glass, etc.
2. This product is used as a polymerization inhibitor. This product is an intermediate used in the preparation of red light sulfide black and acid dyes, photographic drugs, explosives and pesticides such as chloropicrin, etc. Used medicinally as a surgical astringent. Often used for the isolation and purification of organic bases.
3. Dye intermediates. Manufactured such as red light sulfide cyan, acid medium olive GG and acid medium brown R.
4. Used in the manufacture of explosives, dyes, matches, organic synthesis, etc. [23]

 微信扫一扫打赏
微信扫一扫打赏

