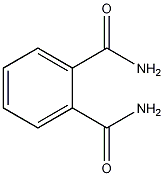
Structural formula
| Business number | 0208 |
|---|---|
| Molecular formula | C8H8N2O2 |
| Molecular weight | 164.16 |
| label |
1,2-Phenyldicarbonamide, Phthalamide, 1,2-Phthalamide, Phthalamide, o-Carbamoylbenzamide, phthalic diamide |
Numbering system
CAS number:88-96-0
MDL number:MFCD00025478
EINECS number:201-870-6
RTECS number:CZ2200000
BRN number:1868220
PubChem ID:None
Physical property data
1. Properties: colorless fine crystals.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 228
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor Pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: When melted, boiling aqueous solution or ethanol solution produces ammonia and phthalimide, the presence of acid can promote its decomposition. Easily soluble in hot organic solvents, slightly soluble in cold water and methanol.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 44.11
2. Molar volume (cm3/mol): 126.8
3. Isotonic specific volume (90.2K ): 356.1
4. Surface tension (dyne/cm): 62.1
5. Polarizability (10-24cm3): 17.48
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 3
6. Topological molecule polar surface area 86.2
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 181
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Colorless fine crystals. When melted, boiling aqueous or ethanol solutions produces ammonia and phthalimide, which can be decomposed in the presence of acid. Easily soluble in hot organic solvents, slightly soluble in cold water and methanol. Melting point 228℃.
Storage method
Should be kept sealed.
Synthesis method
Purpose
Organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

