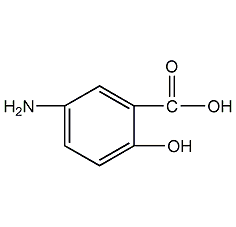
Structural formula
| Business number | 020W |
|---|---|
| Molecular formula | C7H7NO3 |
| Molecular weight | 153.14 |
| label |
5-amino-2-hydroxybenzoic acid, ammonia salicylic acid, 5-Amino-2-hydroxybenzoic acid, 5-AS, Mesalamine, H2NC6H3-2-(OH)CO2H, Digestive system medication |
Numbering system
CAS number:89-57-6
MDL number:MFCD00007877
EINECS number:201-919-1
RTECS number:VO1400000
BRN number:2090421
PubChem number:24890780
Physical property data
1. Properties: White to pink crystals. Sensitive to light and air.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Not determined
5. Boiling point (ºC, normal pressure): Not determined
6. Boiling point (ºC, 5.2kPa): Not determined Determined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturation Vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (% , V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in hot water, soluble in hydrochloric acid , slightly soluble in cold water and ethanol.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 39.30
2. Molar volume (cm3/mol): 102.6
3. Isotonic specific volume (90.2K ): 310.2
4. Surface tension (dyne/cm): 83.3
5. Polarizability (10-24cm3): 15.57
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 4
4. Rotatable chemistry��Number: 1
5. Number of tautomers: 9
6. Topological molecule polar surface area 83.6
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 160
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed and stored at 4℃.
Synthesis method
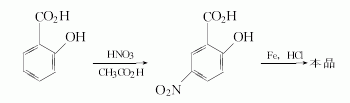
Method 1: After mixing salicylic acid and water, slowly add a mixture of 68% nitric acid and glacial acetic acid dropwise at 50°C with stirring. Then raise the temperature to 70~80℃ for reaction. Pour the reaction solution into water and place it at 5°C. Filter, and the filter cake is recrystallized with hot water to obtain 5-nitrosalicylic acid solid with a yield of 40%.
At 60°C, add iron powder to the hydrochloric acid solution. After heating and boiling, add 1/4 of 5-nitrosalicylic acid. After vigorous stirring, add the remaining nitro compounds and iron powder alternately in batches. After addition, react at 102°C. While hot, use 50% sodium hydroxide to make it strongly alkaline and filter. Wash the filter cake with hot water. After combining the filtrate and washing liquid, add insurance powder and acidify it to Ph value 4 with 40% sulfuric acid. Cool, filter, dry, dissolve it and sodium bisulfite in hot water, and boil with activated carbon. Hot filter and wash the filter cake with a small amount of hot water. The filtrate and washing liquid were combined and placed at 5°C. The crystals were filtered, washed thoroughly with ice water, and dried to obtain white needle-shaped mesalazine crystals with a melting point of 278°C (decomposition).
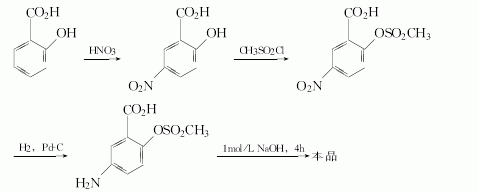
Method 2: Nitration of salicylic acid to obtain 5 – After nitrosalicylic acid, it reacts with methanesulfonyl chloride to protect the phenolic hydroxyl group, then hydrogenates to reduce the nitro group to an amino group, and finally hydrolyzes to obtain mesalazine.
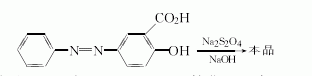
Method 3: Aniline is obtained by diazotization Nitrogen salt, add it to a solution of salicylic acid, sodium carbonate and 40% sodium hydroxide, stir vigorously at 0 to 6°C, and keep the Ph value at 8. Filter, wash with saturated brine, and press dry. Dissolve in 40% sodium hydroxide solution and add insurance powder at 80~85℃. After the solution completely fades, aniline is evaporated by steam distillation and filtered. The filtrate was adjusted to Ph value 6~6.5 with concentrated hydrochloric acid and left overnight. Filter, wash with cold water, and dissolve the dried crude product in water. Add concentrated hydrochloric acid dropwise until dissolved, then add activated carbon and heat to boiling. Filter, cool, and adjust to Ph value 2 to 3 with dilute sodium hydroxide. Filter, wash with cold water, and vacuum dry at room temperature to obtain off-white mesalazine solid with a melting point of 278°C (decomposition) and a total yield of 45.9%.

Method 4: p-aminophenol and appropriate amount of catalyst, at CO2 pressure of 2.0~3.0MPa and 130~190℃ Stir. Cool to room temperature, add water and insurance powder, stir. Cool to 0~3℃, filter out insoluble matter and wash with water. Combine the washing liquid and the filtrate, adjust the pH value to 3 to 4 with hydrochloric acid, filter, and wash with water to obtain crude mesalazine. Add water, sodium bicarbonate and sodium bisulfite to the crude product, heat to 50-60°C until fully dissolved, add activated carbon and boil. Filter, cool, and adjust to Ph value 3.5 with dilute hydrochloric acid. Filter, wash, and dry to obtain gray-white fine product. The yield is 91.1% and the content is 99.2%.
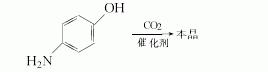
Purpose
1. Dye intermediates. 5-Aminosalicylic acid (the drug name of 5-ASA is Masalazine) is an important pharmaceutical and dye chemical raw material. It is also the main raw material of sulfasalazine, a drug that treats chronic colitis. It itself also has similar Therapeutic effect. 5-Aminosalicylic acid has three active reactive groups, hydroxyl and carboxyl, on the ring, which can carry out a variety of reactions. Therefore, in the dye industry, it can be used to make a variety of reactive dyes with excellent quality. Also used in the manufacture of photosensitive paper. Ulcerative colitis treatments. Manufacture of photosensitive paper, azo and sulfur dyes. intermediate.
2.Ulcerative colitis treatment medicine. It has a significant inhibitory effect on inflammation of the intestinal wall; mesalamine can inhibit the synthesis of prostaglandins that cause inflammation and the formation of inflammatory mediators leukotrienes, thereby significantly inhibiting inflammation of the intestinal mucosa. It is more effective on the connective tissue of the intestinal wall with inflammation. For ulcerative colitis, ulcerative proctitis, and Crohn’s disease.

 微信扫一扫打赏
微信扫一扫打赏

