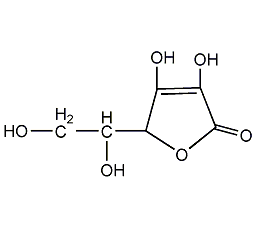
Structural formula
| Business number | 0213 |
|---|---|
| Molecular formula | C6H8O6 |
| Molecular weight | 176.12 |
| label |
D-2,3,5,6-tetrahydroxy-2-hexenoic acid-γ-lactone, Isovitamin C, D-(−)-Isoascorbic acid, D-erythro-Hex-2-enoic acid γ-lactone, Antioxidants |
Numbering system
CAS number:89-65-6
MDL number:MFCD00005378
EINECS number:201-928-0
RTECS number:KF3015000
BRN number:84271
PubChem number:24881081
Physical property data
1. Properties: white shiny granular crystals.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 164~172℃ (decomposition) 5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7 . Refractive index: No determination
8. Flash point (ºC): No determination
9. /p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor Pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, ethanol and pyridine, can Soluble in acetone, slightly soluble in glycerin, insoluble in ether and benzene.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 35.26
2. Molar volume (cm3/mol): 90.1
3. Isotonic specific volume (90.2K ): 310.3
4. Surface tension (dyne/cm): 140.5
5. Polarizability (10-24cm3): 13.97
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): -1.8
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 8
6. Topological molecular polar surface area (TPSA): 107
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 232
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 2
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters : 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is quite stable in the air, but deteriorates rapidly in the presence of air in the solution.
2.This product has better antioxidant properties than ascorbic acid, but poorer heat resistance. It has strong reducing properties and will slowly change color and decompose when exposed to light. Heavy metal ions will promote its decomposition.
Storage method
1. Packed in double-layered food paper or polyethylene plastic bags, with silica gel desiccant in the interlayer, and packed in hard cardboard boxes or barrels.
2. It is strictly prohibited to mix storage with toxic, harmful or other pollutants and oxidizing substances. Store in a dry place, protected from moisture and heat.
Synthesis method
1. It is made from corn starch as raw material and fermented into gluconic acid. The process used by Japan’s Fujisawa Pharmaceutical Company is: glucose is produced from corn starch, then Methly2-ketogluconate is produced, treated with sodium methoxide to obtain sodium erythorbate, and then passed through an ion exchange resin Desalting, erythorbic acid is produced.
2. Two-step method: first biosynthesize 2-keto-D-gluconic acid, and then chemically synthesize D-isoascorbic acid. It can oxidize glucose to produce 2-keto- Staphylococcus aureus, Sescherichia marcescens, Erwinia, etc. After the fermentation is completed, the fermentation broth is neutralized with calcium carbonate, and then classified, separated and extracted. 2-Keto 2-D gluconic acid can be obtained from fermentation broth through free acid extraction or calcium salt extraction.
(1) Free acid extraction
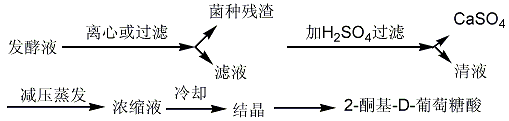
(2) Calcium salt extraction
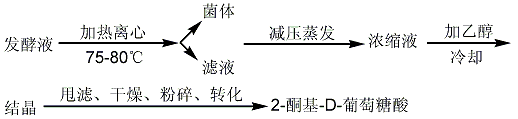
(3) Synthesis of D-erythorbic acid
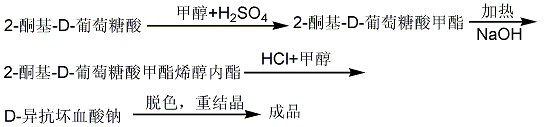 3.Using fermentation method. Glucose is fermented by aerobic fermentation (28°C, 50h) by Pseudomonas fluorescence to obtain α-ketogluconate calcium, which is then used to form methyl ester using methanol and sulfuric acid. It is obtained by adding methanol sodium hydroxide solution for enolization reaction.
3.Using fermentation method. Glucose is fermented by aerobic fermentation (28°C, 50h) by Pseudomonas fluorescence to obtain α-ketogluconate calcium, which is then used to form methyl ester using methanol and sulfuric acid. It is obtained by adding methanol sodium hydroxide solution for enolization reaction.
Purpose
1.D-Erythorbic acid is mainly used in the food industry and pharmaceutical industry. As a food antioxidant, preservative and color development aid, it is widely used in meat products, fish products, beer, fruit juices, fruits, canned vegetables and oils. In practical applications, its sodium salt is almost always used.
2.Mainly used as antioxidants, preservatives, and color development aids. The general dosage (mass fraction) is 0.1% to 0.5%.
3.Isoascorbic acid is mainly used as boiler water, circulating cooling water and other industrial water in the field of water treatment. Deoxidizer.

 微信扫一扫打赏
微信扫一扫打赏

