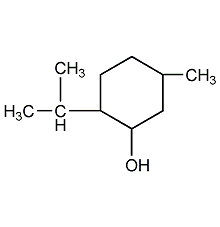
Structural formula
| Business number | 021C |
|---|---|
| Molecular formula | C10H20O |
| Molecular weight | 156.27 |
| label |
2-isopropyl-5-methylcyclohexanol, Menthol (racemic), menthol, 2-Isopropyl-5-methyl cyclohexanol, Menthol (DL), p-Menthan-3-ol, Menthyl alcohol, artificial flavors, alcohol solvent |
Numbering system
CAS number:89-78-1
MDL number:MFCD00001484
EINECS number:201-939-0
RTECS number:OT0350000
BRN number:1902288
PubChem number:24901226
Physical property data
1. Properties: Colorless and transparent rod-shaped crystals with a cool mint aroma.
2. Density (g/mL, 20/4℃): 0.890
3. Relative density (20℃, 4℃): 0.90415
4. Melting point (ºC): 27~28
5. Boiling point (ºC, normal pressure): 212~215
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index (n25D): 1.4615
8. Flash point (ºC): 95
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16 . The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper explosion limit (%, V/V): Undetermined
18. The lower explosion limit (% ,V/V): Undetermined
19. Solubility: Easily soluble in alcohol, chloroform, ether, glacial acetic acid, liquid paraffin, animal oil and essential oil, slightly soluble in water.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 47.83
2. Molar refractive index: 47.83
2. Molar refractive index� Volume (cm3/mol): 175.5
3. Isotonic volume (90.2K): 409.8
4. Surface tension (dyne/cm) :29.7
5. Polarizability (10-24cm3): 18.96
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 3
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 20.2
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 120
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 3
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. The left-handed one is colorless needle crystal. Soluble in ethanol and miscible with oils. It has a cool, fresh and pleasant mint characteristic aroma with a sweet spiciness. It gives people a cold feeling and the fragrance is translucent, but not long-lasting. The taste is also fresh, sweet and cool.
2. Exist in flue-cured tobacco leaves, oriental tobacco leaves, and mainstream smoke.
3. Naturally found in peppermint oil and Japanese peppermint oil, and in small amounts in essential oils such as geranium oil.
4. There are 12 isomers.
5. There is a trace amount of liquid oil and a very small amount of non-volatile matter on the surface of ordinary crystals. Synthetic menthol is a mixture of various isomers.
6. It has certain toxicity, irritation and allergic reactions.
Storage method
This product should be sealed and stored in a cool place.
Synthesis method
1. Menthol can be purified from natural mint crude oil or made synthetically. The essential oil obtained by steam distillation of the above-ground parts (stems, branches, leaves and inflorescences) of the Lamiaceae plant mint is called mint crude oil, with an oil yield of 0.5-0.6. There are many methods for synthesizing menthol.
2. Tobacco: FC, 18; OR, 41. Synthesis: L-menthol is obtained from peppermint oil through cooling, crystallization and separation. (+)-menthol is prepared by a semi-synthetic method, using citronellal obtained by distilling citronella oil or piperone obtained from eucalyptus oil as raw materials.
Purpose
1. Menthol and racemic menthol are both used as flavoring agents in toothpaste, perfume, beverages and candies. It is used as a stimulant in medicine, acting on the skin or mucous membranes, and has a cooling and antipruritic effect; taken orally, it can be used as a carminative for headaches and inflammation of the nose, pharynx, and larynx. Its esters are used in spices and medicines. In the world, my country and Brazil are the main natural mint production gardens, with the annual output of peppermint oil reaching 2000-3000t.
2.Widely used in daily flavors, edible flavors, and tobacco flavors. Generally, it is 1100mg/kg in chewing gum; 400mg/kg in candy; 130mg/kg in baked goods; 68mg/kg in ice cream; soft drinks Medium 35mg/kg.
3. It has bactericidal and antiseptic effects.
4. Used as special additives for therapeutic cosmetics. Used for toilet water, etc. It can also be used as a flavoring agent for toothpaste, perfume, beverages and candies. Used for making candies, beverages, spices and medicinal purposes.

 微信扫一扫打赏
微信扫一扫打赏

