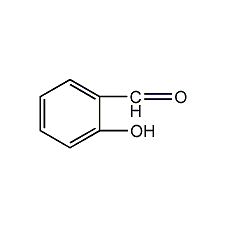
Structural formula
| Business number | 021T |
|---|---|
| Molecular formula | C7H6O2 |
| Molecular weight | 122.12 |
| label |
2-Hydroxybenzaldehyde, o-Hydroxybenzaldehyde, o-formylphenol, 2-Hydroxybenzaldehyde, o-Formylphenol, Salicylal, fungicides, spices, Multifunctional solvent |
Numbering system
CAS number:90-02-8
MDL number:MFCD00003317
EINECS number:201-961-0
RTECS number:VN5250000
BRN number:471388
PubChem number:24845444
Physical property data
1. Properties: colorless, transparent to light yellow oily liquid with burning and almond smell. [1]
2. Melting point (℃): -7[2]
3. Boiling point (℃): 197[3]
4. Relative density (water = 1): 1.17[4]
5. Relative vapor Density (air=1): 4.2[5]
6. Saturated vapor pressure (kPa): 0.13 (33℃)[6]
7. Heat of combustion (kJ/mol): -3328.9[7]
8. Critical pressure (MPa): 4.99[8]
9. Octanol/water partition coefficient: 1.7~1.81[9]
10. Flash point (℃): 77.8 ( CC)[10]
11. Ignition temperature (℃): 249[11]
12. Solubility : Used as analytical reagents, spices, gasoline additives and in organic synthesis. [12]
Toxicological data
1. Acute toxicity[13] LD50: 520mg/kg (rat oral); 3000mg/kg (rabbit transdermal)
2. Irritation[14] Rabbit transdermal: 500mg (24h), moderate irritation.
Ecological data
1. Ecotoxicity[15] IC50: 1.6~4.9mg/L (72h) (algae)
2. Biodegradability No data available
3. Non-biodegradability No data available
4. Other harmful effects[16] This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 34.88
2. Molar volume (cm3/mol): 99.5
3. Isotonic specific volume (90.2K ): 267.3
4. Surface tension (dyne/cm): 52.0
5. Polarizability (10-24cm3): 13.83
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.8
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Tautomeric��Number: 5
6. Topological molecular polar surface area (TPSA): 37.3
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 101
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. The number of determined chemical bond stereocenters: 0
14. The number of uncertain chemical bond stereocenters: 0
p>
15. Number of covalent bond units: 1
Properties and stability
1. Stability[17] Stable
2. Incompatible substances[18] Strong oxidants, strong acids, strong alkali
3. Polymerization hazard[19] No polymerization
Storage method
Storage Precautions[20] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, acids, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1.Mix phenol and excess sodium hydroxide to prepare a sodium phenolate solution and heat to 60 ~65°C, slowly add chloroform. The reaction will be purple at first and gradually turn to orange-red. After the reaction is completed, the excess chloroform will be evaporated with water vapor. After the generated salicylaldehyde sodium salt is neutralized with acid, water vapor will be passed through it. Steam out the salicylaldehyde.
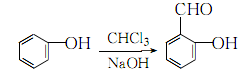
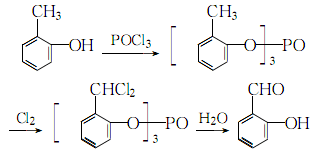
2. After mixing o-cresol and phosphorus oxychloride, they react at a certain temperature to generate tri-o-cresol phosphate. Then chlorine gas is introduced until α-α-dichloro derivatives are generated. Finally, a high-yield and high-content salicylaldehyde product is obtained through hydrolysis and distillation. The main reactions are as follows:
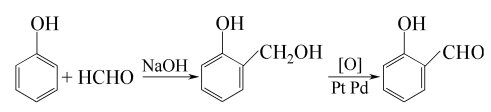
3. Salicin method, that is, salicin (or salicyl alcohol) is produced by air oxidation in the presence of platinum or palladium catalyst. It can also be prepared from phenol as starting material. The reaction formula is as follows:
Purpose
1. Mainly used to produce coumarin, prepare violet spices, and can also be used as a fungicide. Determination of hydrazine, photometric determination of scandium. Extraction and separation of nickel and cobalt. Precipitating agent for nickel, copper, iron, palladium and vanadium. Solvents, fragrances and organic synthesis intermediates.
2. Salicylaldehyde has good bactericidal effect and is used as a bactericide in water treatment such as industrial circulating cooling water and oil field reinjection water. Used as antiseptic and fungicide in oil fragrances. When the concentration is 50mg/L, it can kill 60% of iron bacteria and 85% of sulfate reducing bacteria. The general dosage for water treatment is 50~100Mg/L. The concentration used should be within its water-soluble range and can be added directly.
3. Used as analytical reagents, spices, gasoline additives and in organic synthesis. [21]

 微信扫一扫打赏
微信扫一扫打赏

