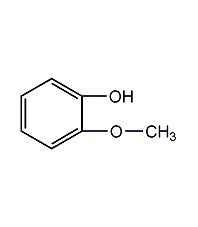
Structural formula
| Business number | 021V |
|---|---|
| Molecular formula | C7H8O2 |
| Molecular weight | 124.14 |
| label |
o-Methoxyphenol, Methylcatechol, 2-Methoxyphenol, Guaiacol, O-methoxyphenol, Methyl catechol, 2 – Methoxyphenol, Guaiacol, natural flavors |
Numbering system
CAS number:90-05-1
MDL number:MFCD00002185
EINECS number:201-964-7
RTECS number:SL7525000
BRN number:508112
PubChem number:24895212
Physical property data
1. Properties: White or slightly yellow crystals or colorless to light yellow transparent oily liquid (above 28℃), the color gradually deepens when oxidized in the air or under sunlight. It has the smell of phenol, as well as smoky, spicy, medicinal, and meaty aromas. 2. Density (g/mL, 25/4℃): 1.1293. Relative vapor density (g/mL, air=1): 1.112~1.143 4. Melting point (ºC): 27~29
5. Boiling point (ºC , normal pressure): 205
6. Boiling point (ºC, 3.2kPa): 107
7. Refractive index (n35D): 1.5341 8. Flash point (ºC): 82.2
9 . Relative density (20℃, 4℃): 1.107647.2
10. Relative density (25℃, 4℃): 1.061480
11. Refractive index at room temperature (n20): 1.531845
12. Refractive index at room temperature (n25): 1.521366
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water and Benzene is easily soluble in glycerol and miscible with ethanol, ether, chloroform, glacial acetic acid and most organic solvents.
Toxicological data
Acute poisoning caused by accidental ingestion.Symptoms include headache, dizziness, fatigue, bluish oral mucosa, metallic taste in the mouth, blue gums and tongue, diarrhea, abdominal cramps, black stools, coma, spasm, and decreased blood pressure in severe cases. Skin contact can cause dermatitis, ulcers, etc.
Ecological data
None
Molecular structure data
Molecular property data:
1. Molar refractive index: 34.81
2. Molar volume (cm3/mol): 111.8
3. Isotonic specific volume (90.2K): 278.9
4. Surface tension (dyne/cm): 38.6
5. Polarizability (10– 24cm3): 13.80
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 1.3
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 3
6. Topological molecular polar surface area (TPSA): 29.5
p>
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 83
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Colorless to light yellow liquid with a burnt-sweet woody aroma.
Easily soluble in ethanol and organic solvents, slightly soluble in water, with a content of more than 99%.
2.Has strong phenol properties and moderate toxicity. Irritating to skin. Taking a large amount can irritate the esophagus and stomach, cause heart failure, collapse and death. Rat oral LD50: 725mg/kg, subcutaneous injection LD50: 900mg/kg. For details on its toxicity and protection methods, see Phenol.
3. Exists in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves and smoke.
4. Naturally found in rue oil, celery seed oil, tobacco leaf oil, orange leaf distillate and castoreum.
Storage method
1. Light-sealed packaging. Store in a cool, dark place.
2. Packed in iron drums, with a net weight of 200kg per drum. Store and transport according to regulations on toxic substances.
Synthesis method
1. It can be isolated from guaiac resin, Xia Yun sesame oil and tobacco leaf oil. This product is obtained by dry distillation of wood, pine tar is treated with alkali to separate the phenolic compounds, and then fractionated and refined to produce natural guaiacol.
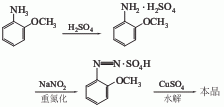
2.O-methoxyanisole diazo method is used. It is obtained from o-methoxyanisole as raw material through diazotization, hydrolysis reaction, extraction and fractionation. It can also be obtained by dry distillation of wood using the wood distillation method.
3. Tobacco: BU, 56; OR, 26; FC, OR, 18.
Purpose
1. It can be used to prepare flavors for food, daily chemicals, tobacco, etc. It is also very useful in medicine and is an intermediate for synthetic spices and drugs.
2.Cosmetic antioxidant. It is a phenolic antioxidant and has a strong antioxidant effect, but generally the amount added should not be too much, and it usually needs to be used in conjunction with synergists, metal ion chelating agents, etc.
3. It is an important raw material for the synthesis of vanillin.
4. In medicine, it has the effect of eliminating phlegm and preventing abnormal fermentation in the intestines and stomach.

 微信扫一扫打赏
微信扫一扫打赏

