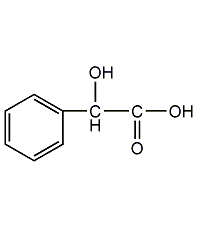
Structural formula
| Business number | 022M |
|---|---|
| Molecular formula | C8H8O3 |
| Molecular weight | 152.15 |
| label |
DL-mandelic acid, 2-Hydroxy-2-phenylacetic acid, α-Hydroxyphenylacetic acid, phenyl glycolic acid, α-Hydroxybenzeneacetic acid, DL-Mandelic acid, Aromatic carboxylic acids and their derivatives, acidic solvent |
Numbering system
CAS number:90-64-2
MDL number:MFCD00064250
EINECS number:202-007-6
RTECS number:OO630000
BRN number:510011
PubChem ID:None
Physical property data
1. Properties: White orthorhombic flake crystals
2. Density (g/mL, 25/4℃): 1.30
3. Relative vapor density (g/ mL, air = 1): Undetermined
4. Melting point (ºC): 119
5. Boiling point (ºC, normal pressure): 321.8
6 . Boiling point (ºC, 5.2kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): 162.6
9. Specific rotation (º C=1, WATER): +156.57°
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, ethanol, ether, and isopropyl alcohol.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 38.90
2. Molar volume (cm3/mol): 115.1
3. Isotonic specific volume (90.2K ): 321.0
4. Surface tension (dyne/cm): 60.4
5. Polarizability (10-24cm3): 15.42
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 57.5
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 138
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Found in burley tobacco leaves.
Storage method
This product should be kept sealed. Packed in 25kg cardboard drum; during transportation, it must be strictly protected from moisture, heat and sunlight; it should be stored in a cool, dry and ventilated place, away from fire and heat sources.
Synthesis method
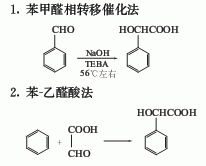
1. Use acetophenone, a by-product of cumene production of phenol, as raw material, chlorine it to obtain dichloroacetophenone, then react with dilute alkali and hydrolyze to obtain mandelic acid. 2. Use benzaldehyde as raw material. Dissolve sodium cyanide in water, add benzaldehyde, and slowly add the saturated solution of sodium bisulfite while stirring. When the addition is half done, add crushed ice and separate the precipitated phenylhydroxyacetonitrile layer from the water layer. The water layer was extracted with benzene, and the benzene was evaporated. The residue was combined with the benzene hydroxyacetonitrile layer, hydrochloric acid was added, and hydrolysis was carried out under cooling for 12 hours. Then heat to remove excess water and hydrochloric acid, cool, and filter out the mixture of ammonium chloride and mandelic acid. The filtrate was evaporated to dryness, and the residue was combined with the above solid and washed with cold benzene. It is then extracted with hot benzene, and the extract is cooled, crystallized, filtered, and dried to obtain mandelic acid with a yield of 50-52%. 3. Benzene-glyoxylic acid method. 4. Acetophenone method: prepared by reacting with nitrobenzene under alkali catalysis.
2. Tobacco: BU, 26 .
3. Preparation method:
Mandelonitrile (3): In a reaction bottle equipped with a stirrer and thermometer, add 350mL of water and 100g of sodium cyanide (2.0mol). Stir to dissolve, add 212g (2.0mol) of newly distilled benzaldehyde (2), add about 300mL of saturated sodium bisulfite solution under vigorous stirring, control the reaction temperature at 30~35°C, and finish adding in 20 minutes (ice can be used water bath cooling). After the addition was completed, the reaction was stirred for 0.5 h. After standing, the upper orange-red oily substance was separated to obtain about 220g of crude mandelonitrile (3). Mandelic acid (1): equipped with a stirrer. In the reaction bottle of the thermometer, add 300 mL of concentrated acetic acid, heat to about 60°C, and add 220 g of the above mandelonitrile (3) in batches (note that the reaction should be carried out in a fume hood). After the reaction was stable, the reaction was heated in a boiling water bath for 2 hours. Cool and leave overnight. Suction filter the precipitated solid (containing ammonium chloride). Extract the solid material twice with diethyl ether ① (400mL×2), and concentrate the diethyl ether solution to about 1/3 volume. Cool and a solid precipitates. Filter and dry to obtain 135g of mandelic acid (1). The yield is 44%. Recrystallize with hot water, mp121~123℃. MP118℃ has also been reported. Note: ① It can also be extracted with hot benzene. [1]
Purpose
In the pharmaceutical industry, it can be used as an intermediate for cefadroxyzole, vasodilator cyclomandelate, eye drops oxybenzole, pemoline, etc., and can also be used as a preservative.

 微信扫一扫打赏
微信扫一扫打赏

