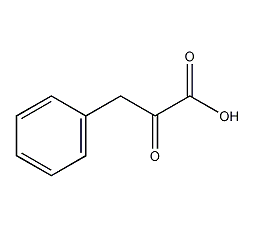
Structural formula
| Business number | 03ZG |
|---|---|
| Molecular formula | C9H8O3 |
| Molecular weight | 164.16 |
| label |
2-Oxophenylpropionic acid, 2-Oxophenylpropionic acid, Aromatic carboxylic acids and their derivatives, acidic solvent |
Numbering system
CAS number:156-06-9
MDL number:MFCD00002589
EINECS number:205-874-1
RTECS number:None
BRN number:2207312
PubChem number:24857257
Physical property data
1. Properties: white crystal.
2. Melting point (ºC): 150~154
3. Solubility: soluble in alcohol, ether, benzene and other solvents.
4. Boiling point (ºC): 299.1
5. Flash point (ºC): 148.9
6. Relative density (d20
sup>4) :1.257
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 42.02
2. Molar volume (cm3/mol): 130.5
3. Isotonic specific volume (90.2K ): 350.2
4. Surface tension (dyne/cm): 52.0
5. Dielectric constant: not available
6. Dipole moment (10 -24cm3): Not available
7. Polarizability: 16.66
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.3
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 2
6. Topological molecule polar surface area 54.4
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 180
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Found in tobacco leaves.
Storage method
Storage temperature -18ºC
Synthesis method
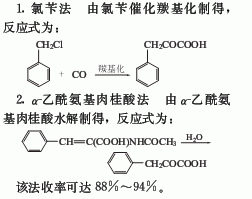
3. Preparation method:
2-Methyl-4-benzylidene-5-oxo-4,5-dihydroxazole (3): Add 58.5g (0.5mol) of acetamidoacetic acid (2) into a 1L reaction bottle, none 30g sodium acetate (0.37mol), 79g newly distilled benzaldehyde (0.74mol), 134g 95% acetic anhydride, install a reflux condenser, and warm with shaking for 10 to 20 minutes. Let it dissolve. Then reflux the reaction for 1 hour. Once cooled, place in the refrigerator overnight. The resulting yellow solid was treated with 125 mL of cold water, crushed, filtered, washed thoroughly with cold water, and placed in a vacuum dryer containing phosphorus pentoxide.After drying, 69 to 72 g of crude compound (3) was obtained, mp was 148 to 150°C, and the yield was 74% to 77%. No further purification is required and can be used directly in the next step. a – Acetamidocinnamic acid (4): Add 47g (0.25mol) of the above compound (3), 450mL acetone and 175mL water to a 1L reaction bottle, and heat to reflux for 4 hours. After most of the acetone is evaporated, add 400 mL of water and continue refluxing for 5 minutes to complete the hydrolysis. Filter, and wash the filter residue twice with hot water. Combine the filtrate and washing liquid and decolorize with activated carbon. Place it in the refrigerator overnight, filter with suction, wash with water, and dry at 90 to 100°C to obtain 41 to 46 g of colorless needle crystals (4), mp 191 to 192°C, and a yield of 80% to 90%. Phenylpyruvic acid (1): Add 10g (0.05mol) of compound (4) and 200mL of 1mol/L hydrochloric acid into a 500mL round-bottom flask, heat and boil for 3 hours. If there is a very small amount of light green oil, it can be filtered out. Cool and crystallize. Filter with suction, wash with cold water, combine the filtrate and washing liquid, and extract with ether 4 times, 50 mL each time. The ether was evaporated to obtain part of the solid. The solids obtained twice were combined and dried in a vacuum dryer containing calcium chloride and sodium hydroxide to obtain 7.2 to 7.7 g of compound (1), mp 150 to 154°C, and a yield of 88% to 94%. [1]
Purpose
Used as a raw material for the synthesis of L-phenylalanine, it can also be used in the preparation of sweeteners (asparti), food nutritional supplements and various anti-cancer drugs .

 微信扫一扫打赏
微信扫一扫打赏

