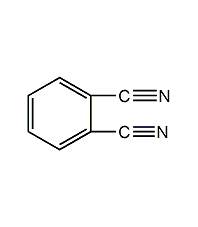
Structural formula
| Business number | 0236 |
|---|---|
| Molecular formula | C8H4N2 |
| Molecular weight | 128.13 |
| label |
1,2-dicyanobenzene, o-Dicyanobenzene, 1,2-Dicyanobenzene, C6H4(CN)2 |
Numbering system
CAS number:91-15-6
MDL number:MFCD00001771
EINECS number:202-044-8
RTECS number:TI8575000
BRN number:775028
PubChem number:24850384
Physical property data
1. Properties: Colorless needle-like crystals. Can evaporate with water vapor. It can be gradually hydrolyzed into acid by hot hydrochloric acid.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 141
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): 162
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in ethanol, ether, chloroform and benzene, Slightly soluble in water and petroleum.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 51.54
2. Molar volume (cm3/mol): 110.0
3. Isotonic specific volume (90.2K ): 300.6
4. Surface tension (dyne/cm): 55.6
5. Polarizability (10-24cm3): 14.22
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 47.6
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 178
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Storage method
Should be sealed and stored in a cool place.
Synthesis method
After phthalimide reacts with ammonia to obtain phthalic acid amide, it is then dehydrated to obtain phthalonitrile. In addition, this product can be obtained by ammoniating phthalic anhydride and then reacting with phosphorus oxychloride, or by ammoxidizing naphthalene pentaamine, or by ammoxidizing o-xylene and ammonia.
Purpose
Used in dyes; pigments and other organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

