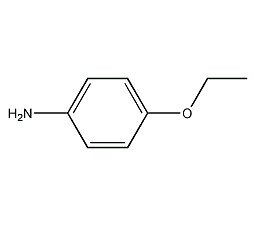
Structural formula
| Business number | 03ZN |
|---|---|
| Molecular formula | C8H11ON |
| Molecular weight | 137.18 |
| label |
4-Aminophenetole, 1-Amino-4-ethoxybenzene, 4-Aminoethoxybenzene, p-Ethoxyaniline, 4-Aminophenylene ether, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:156-43-4
MDL number:MFCD00007865
EINECS number:205-805-5
RTECS number:SI6465500
BRN number:606666
PubChem number:24898024
Physical property data
1. Properties: colorless oily flammable liquid
2. Density (g/mL, 25/4℃): 1.0652
3. Refractive index (n D20): 1.5609
4. Flash point (℃): 115.6
5. Melting point (℃): 2.4
6. Boiling point ( ºC): 253~255
7. Boiling point (ºC, 1.33kpa): 103~104
8. Heat of vaporization (KJ/mol): 49.14
9. Vapor pressure (Pa, 25ºC): 2.3598
10. Solubility: Almost insoluble in water and inorganic acids, soluble in ethanol, ether, and chloroform.
Toxicological data
1. Acute toxicity: Highly toxic. The LD50 given by oral administration to mice was 540 mg/kg; the LD50 given to rats was 580 mg/kg. The maximum allowable concentration in the air is 0.2mg/m3.
Ecological data
None
Molecular structure data
1. Molar refractive index: 41.80
2. Molar volume (cm3/mol): 132.2
3. Isotonic specific volume (90.2K ): 329.5
4. Surface tension (dyne/cm): 38.5
5. Dielectric constant: not available
6. Dipole moment (10 -24cm3): Not available
7. Polarizability: 16.57
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 35.2
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 87.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Colorless oily flammable liquid. Gradually turns red to brown on exposure to air and sunlight. Insoluble in water and inorganic acids, soluble in ethanol, ether and chloroform, etc.
Decomposes when heated to produce toxic gases. It is toxic and can be absorbed through the skin, causing poisoning symptoms similar to aniline, such as headache, dizziness, cyanosis, etc.
Storage method
Stored in a cool and ventilated warehouse, away from fire, heat sources, direct sunlight, and isolated from edible raw materials for storage and transportation.
Synthesis method
1. Obtained from the reduction of p-nitrophenylethyl ether ([100-29-8]). Add sodium sulfide and sulfur to water and raise the temperature to 138-142°C. Let the solution stand for clarification and then add it to the reduction pot. Heat and stir, add p-nitrophenylethyl ether uniformly and slowly within 5-6.5 hours, react at 132-134°C under normal pressure for 3 hours, and at 138-140°C under pressure (below 0.1MPa) for 10-12 hours. Add water, stir and let stand for 1 hour. Separate the lower sodium thiosulfate mother liquor, separate the upper oil layer to clean the residue, distill under reduced pressure, and collect the 168-170°C (5.33kPa) fraction to obtain p-ethoxyaniline with a content of 99%. The yield is over 95%. Hydrogenation reduction can also be used, using nickel as a catalyst to react with hydrogen at 130-140°C and a pressure of 0.6MPa, and p-nitrophenylethyl ether is reduced to p-ethoxyaniline. In addition, using nitrobenzene as the raw material, in the presence of sulfuric acid, using nickel oxide as the catalyst and dimethyl sulfoxide as the accelerator, it is ethoxylated with absolute ethanol and reduced in one step to prepare p-ethoxyaniline.
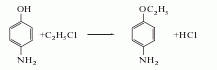
2. Para-aminophenol method Produced by reaction with ethyl chloride.
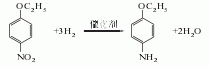
Purpose
1. Used in the production of rubber antioxidant AW, namely 6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline. It is also used in feed and food to prevent fat and protein from oxidizing and deteriorating during storage. It is also used for the preservation of vitamin A, vitamin E and other medicines. When used as an antioxidant in feed and food, it is called ethoxyquin ([91-53-27]). In medicine, it is used to produce antipyretic and analgesic drugs phenacetin, antiseptic and antiseptic drugs Rivanol.
2. Used to prepare dyes such as naphthol AS-VL, alizarin red 5G and fast acid blue R.
3. Used to determine copper, iron, manganese, vanadium, zinc, chromate and cyanide. Redox indicator.

 微信扫一扫打赏
微信扫一扫打赏

