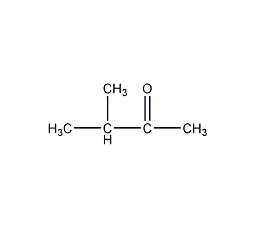
Structural formula
| Business number | 05T2 |
|---|---|
| Molecular formula | C5H10O |
| Molecular weight | 86.13 |
| label |
3-methylbutanone, Isopropyl methyl ketone, Isopropyl methyl ketone, Methyl isopropyl ketone, Pesticide research and development |
Numbering system
CAS number:563-80-4
MDL number:MFCD00008919
EINECS number:209-264-3
RTECS number:EL9100000
BRN number:1071238
PubChem number:24869942
Physical property data
1. Properties: colorless liquid with acetone odor. [1]
2. Melting point (℃): -92[2]
3. Boiling point (℃): 93~95[3]
4. Relative density (water=1): 0.81[4]
5. Relative vapor density (air=1): 2.97[5]
6. Saturated vapor pressure (kPa): 5.5 (20℃)[6]
7. Critical pressure (MPa): 3.8[7]
8. Octanol/water partition coefficient: 0.84[8]
9. Flash point (℃): 6 (CC) [9]
10. Ignition temperature (℃): 475 [10]
11. Explosion upper limit (%): 8.0[11]
12. Explosion lower limit (%) : 1.2[12]
13. Solubility: Slightly soluble in water, soluble in most organic solvents. [13]
14. Specific heat capacity (KJ/(kg·K), 20ºC): 2.076
15. Thermal conductivity (W/(m ·K), 20ºC): 0.1419
16. Relative density (25℃, 4℃): 0.7982
17. Refractive index at room temperature (n25 ): 1.3857
18. Critical density (g·cm-3): 0.28
19. Critical volume (cm3·mol-1): 308
20. Critical compression factor: 0.255
21. Eccentricity factor: 0.350
22 .Solubility parameter (J·cm-3)0.5: 18.253
23. van der Waals area (cm2·mol-1): 8.530×109
24. van der Waals volume (cm3·mol -1): 59.490
25. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3134.13
26. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -262.57
27. Gas phase standard entropy (J·mol-1·K-1): 369.9
28. Gas phase standard free energy of formation (kJ·mol-1): -139.12
29. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -3097.23
30. Liquid phase standard claimed heat (enthalpy) ( kJ·mol-1): -299.47
31. Liquid phase standard entropy (J·mol-1·K-1): 268.5
32. Liquid phase standard formation free energy (kJ·mol-1): -145.77
33. Liquid phase standard Hot melt (J·mol-1·K-1): 179.5
Toxicological data
1. Acute toxicity[14] LD50: 148mg/kg (rat oral); 6350mg/kg (rabbit dermal )
2. Irritation[15]
Rabbit transdermal: 500mg ( 24h), moderate stimulation�
Rabbit eye: 100mg (24h), mild irritation.
Ecological data
1. Ecotoxicity[16] LD50: 2g/L (24h) (fish)
2. Biodegradability No information available
3. Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 25.20
2. Molar volume (cm3/mol): 108.5
3. Isotonic specific volume (90.2K ): 233.5
4. Surface tension (dyne/cm): 21.4
5. Polarizability (10-24cm3): 9.99
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 3
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 55
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[17] Stable
2. Incompatible substances [18] Strong oxidizing agent, strong alkali, strong reducing agent
3. Polymerization hazard[19] No aggregation
Storage method
Storage Precautions[20] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature does not exceed 37℃. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Add an equal mole of bromine dropwise to tert-amyl alcohol at 50-60°C to prepare 2,3-dibromoisopentane, then add water and reflux for 3-5 hours until the hydrolysis is complete, and then evaporate from the reaction mixture The crude product 3-methyl-2-butanone was refined to obtain the finished product with a yield of 59%.
2. Preparation method:
In a 1.5L reaction bottle equipped with a stirrer, thermometer, and dropping funnel, add redistilled (boiling point range 0.5°C) 2-tert. Take 176.0g (2mol) of pentanol (2) and heat it to 50-60°C in a water bath. Add 320g (2mol) of bromine dropwise within 21 hours. After the addition, continue to stir the reaction until the color of bromine disappears. Generates trimethyldibromoethane (3). Add 540 mL of water to the reaction bottle, install a reflux condenser, and heat to reflux for 3 to 5 hours until hydrolysis is complete. Change to a distillation device for direct distillation until the temperature rises and no oil is distilled. Continue distillation of the residue to obtain approximately 380 mL of azeotropic hydrobromic acid. The distillate was neutralized with sodium carbonate until alkaline, and the organic layer was separated. Add 20g of powdered potassium carbonate and 5mL of water to the organic layer, and heat to reflux for 16h. Separate the oil layer and dry it with anhydrous calcium chloride or anhydrous potassium carbonate. Place it in a distillation bottle containing 2g of dry sodium carbonate for fractionation, and collect the fractions at 92-94°C to obtain 102g of slightly yellow liquid compound (1), with a yield of 59%. [22]
Purpose
Used as solvent, dewaxing agent, and also used in organic synthesis. [21]

 微信扫一扫打赏
微信扫一扫打赏

