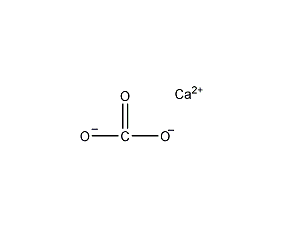
Structural formula
| Business number | 050S |
|---|---|
| Molecular formula | CaCO3 |
| Molecular weight | 100.09 |
| label |
light calcium carbonate, precipitated calcium carbonate, colloidal calcium carbonate, calcium carbonate whiskers, calcite, Precipitated calcium carbonate, Colloidal calcium carbonate, leavening agent, treatment agent, anticaking agent, regulator, enhancer, Hardener, Raw materials and intermediates used in ink |
Numbering system
CAS number:471-34-1
MDL number:MFCD00010906
EINECS number:207-439-9
RTECS number:FF9335000
BRN number:8008338
PubChem ID:None
Physical property data
1. Properties: White fine crystalline powder, odorless and tasteless, can absorb odor. 2. Relative density (g/ m3, 25/4℃): 2.6-2.7 (2.710-2.930, heavy calcium carbonate)
3. Relative vapor density (g/cm3, air=1): 2.5~2.74. Melting point (ºC): 1339℃ 825-896.6 (decomposition, light calcium carbonate) 5. Boiling point (ºC, normal pressure): Undetermined 6. Boiling point (ºC, 5.2kPa): Not determined 7. Refractive index: 1.498. Flash point (ºF): 1389. Specific rotation (º): Not determined 10. Autoignition point or ignition temperature (ºC): Not determined 11. Vapor Pressure (kPa, 25ºC): Undetermined 12. Saturated vapor pressure (kPa, 60ºC): Undetermined 13. Heat of combustion (KJ/mol): Undetermined 14. Critical temperature (ºC): Undetermined 15. Critical pressure (KPa ): Undetermined 16. Log value of oil-water (octanol/water) partition coefficient: Undetermined 17. Explosion upper limit (%, V/V): Undetermined 18. Explosion lower limit (%, V/V): Undetermined 19 . Solubility: Soluble in dilute acids such as acetic acid and hydrochloric acid, insoluble in dilute sulfuric acid, and almost insoluble in water and ethanol. 20. Specific heat capacity (J/(g·℃)): 0.836~0.8951 (0~100℃) 21. Linear thermal expansion coefficient (℃): 11.7×10-6 (15~100℃)
Toxicological data
Acute toxicity: LD50: 6450mg/Kg (Dabai�, dissolve 1 part of ammonium carbonate (chemically pure) with 4 parts of distilled water, filter, and slowly add to the calcium nitrate aqueous solution heated to 60°C while stirring:
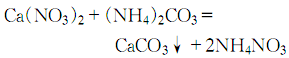
When the reaction is completed, let it stand for complete precipitation. Pour out the solution, filter it, and slowly wash it with distilled water until the NO3– ion content is verified. Dry at 110°C to obtain reagent calcium carbonate. If high-purity calcium nitrate and ammonium carbonate are used, and conductive water is used, spectrally pure calcium carbonate can be obtained by removing the water on an electric stove or in an oven and holding it at a constant temperature of 220°C for 3 to 4 hours.
8.Heavy calcium carbonate mainly uses the replacement method. A calcium chloride solution of a certain concentration is added to a sodium carbonate solution of a certain concentration to perform a displacement reaction, and then undergoes precipitation, separation, drying, crushing, and sieving to obtain it.
9.Natural calcium carbonate mainly uses the crushing method. It is obtained by coarsely crushing, finely crushing and sifting natural whitestone ore containing more than 90% calcium carbonate.
Purpose
1. Light calcium carbonate can be used as a leavening agent, flour treatment agent, anti-caking agent, acidity regulator, nutritional fortifier, solidifying agent, etc. Our country stipulates that it can be used in various foods and gum bases that require the addition of puffing agents, in accordance with GMP. It can also be used as flour improver, with a maximum usage of 0.03%; milk powder 7.5~18g/kg; soy milk powder and soy flour 4~20g/kg; soft drinks 0.4~3.4g/kg; lotus root starch 6~8g/kg; ready-to-eat breakfast Cereal products 2~7g/kg. FDA (184.1192, 2000) does not make restrictive regulations, and it follows GMP. When this product is used as a leavening agent, it is often used in conjunction with other varieties. The leavening agent obtained by compounding it with sodium bicarbonate, alum, etc., slowly releases carbon dioxide when heated, making the food produce a uniform and delicate leavening body. It can improve the quality of pastries, bread and biscuits. In addition, it also has the effect of strengthening calcium. The smaller the calcium carbonate particles, the easier it is to absorb. In Japan, light calcium carbonate is used as a leavening agent, and the dosage in general food is 1%.
2. Used as feed nutritional enhancer.
3. Calcium carbonate whiskers are one of the earliest and largest fillers used in the rubber and plastic industries. In the rubber industry, it is widely used in hoses, rubber sheets, tapes, rubber shoes and medical products. It is a good filler to reduce the cost of products. In the plastics industry, it is mainly used in soft polyvinyl chloride compounds, plastisols and glass fiber reinforced polyester compounds, such as wire sheaths, artificial leather and other extruded and calendered products. The general dosage is about 20 parts. It is also widely used in calcium plastic materials, such as rigid polyvinyl chloride pipes, plates, etc. In addition, this product is also widely used in papermaking, coatings, ink industries and daily chemical products.
4. Compared with general granular calcium carbonate, calcium carbonate whiskers show significant strengthening and toughening effects. Calcium carbonate whiskers can be used to reinforce plastics and rubber. For example, it can be used as a reinforcing modifier for polyamide, polycarbonate, thermoplastic polyester, ABS, PP and other resins, which can not only reduce costs, but also improve the bending strength, dimensional stability and thermal stability of the product. It is used as high-grade paper filler in the papermaking industry. This paper can be used as flame-retardant paper for interior decoration and other aspects. Calcium carbonate whiskers are non-toxic and are an ideal substitute for asbestos. They can be used as friction materials instead of asbestos to make friction materials such as automobile brake pads.
5.It is the filler of rubber, which can make the rubber bright in color, high in elongation, high in tensile strength and good in wear resistance. It is also used as filler in industries such as artificial leather, wires, polyvinyl chloride, coatings, inks and papermaking. When used in the production of microporous rubber, it can foam evenly.
6.Used as analytical reagents, such as reference reagents. Sample flux and volatile additives in emission spectrum analysis. It is also used as silicon single crystal slicing glue, thick film capacitor material, optical glass and pharmaceutical industry raw materials.
7.Used as a white filler for plastics, paper, rubber, coatings, inks, etc., it is the most widely used filler one. It is low in price, widely available in sources, and has low relative density. In addition to its incremental effect, it can also improve processing performance and product performance. In adhesive manufacturing, it can be used as a filler for epoxy glue, nylon sealant, chloroprene sealant, etc. In some soft polyvinyl chloride, it has thermal stabilization effect, which can reduce the amount of heat stabilizer. Used in polyvinyl chloride paste as a viscosity regulator. Used in wire coverings, artificial leather, etc. to improve the color stability of products. In the daily chemical industry, it is used as raw material for manufacturing toothpaste, tooth powder, etc. It is used medicinally as an antacid for gastric and duodenal ulcers and an antidote for acidosis. In agriculture, it is used as dairy cattle feed additive and antibacterial agent. Used in industrial waste gas treatment to remove SO2 from sulfur-containing waste gas. Used as neutralizing agent and flocculant for industrial wastewater in water treatment. Used as anti-adhesive agent in the production of linoleum felt. It is used to eliminate sourness in wine making and is also used as filler in the manufacture of matches, pencils, crayons, insulation materials, welding rods, etc.
8. Used to make raw materials such as powder, powder cake, gouache, rouge, etc., and used as a flavor mixture when manufacturing powder cosmetics , it is also mainly used as a friction agent in toothpaste and is a hard abrasive used in toothpaste.
�hard abrasives.

 微信扫一扫打赏
微信扫一扫打赏

