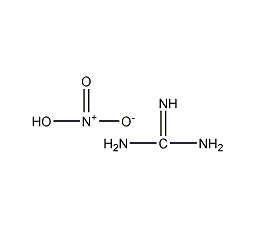
Structural formula
| Business number | 059Q |
|---|---|
| Molecular formula | CH6N4O3 |
| Molecular weight | 122.08 |
| label |
Guanidinium nitrate |
Numbering system
CAS number:506-93-4
MDL number:MFCD00013028
EINECS number:208-060-1
RTECS number:None
BRN number:3596600
PubChem number:24873458
Physical property data
1. Character: white granules[1]
2. Melting point (℃): 213~215[2]
3. Octanol/water partition coefficient: -8.35[3]
4. Solubility: soluble in water, soluble in ethanol, slightly soluble in acetone, insoluble In benzene, ether. [4]
Toxicological data
1. Skin/eye irritation data: Standard Draize test rabbit direct contact with skin: 500mg/24 HREACTION SEVERITY: severe; standard Draize test rabbit direct contact with eyes: 92mgREACTION SEVERITY: mild;
2. Acute Toxicity: Rat oral LD50: 730mg/kg, altered sleep time (including altered righting reflex), muscle contraction or spasm, irritability; Mouse oral LD50: 1028mg/kg, altered sleep duration (including altered righting reflex) Reflex), excitement, changes in body activity (specific analysis); Rabbit transdermal LD50: >2gm/kg, skin and appendages – dermatitis, others (post systemic exposure).
3. Acute toxicity [5] LD50: 730mg/kg (rat oral)
4 . Irritation[6]
Rabbit transdermal: 500mg, severe irritation.
Rabbit eye: 92mg, mild irritation.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[7] This substance is harmful to the environment and should be specially Pay attention to water pollution.
Molecular structure data
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 142
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 51
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
1. Stability[8] Stable
2. Incompatible substances[9] Strong reducing agents, flammable or combustible substances, sulfur, phosphorus
3. Conditions to avoid contact [10] Heat, friction , vibration, impact
4. Aggregation hazards[11] No aggregationp>
5. Decomposition products[12] Nitrogen oxides
Storage method
Storage Precautions[13] Store in a cool, ventilated warehouse. The storage temperature does not exceed 30°C and the relative humidity does not exceed 80%. Keep away from fire and heat sources. The packaging is sealed. It should be stored separately from flammable (combustible) materials, reducing agents, etc., and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
The preparation method is the reaction of dicyandiamide and ammonium nitrate. Dicyandiamide and ammonium nitrate are condensed at a ratio of 1:2 at 120 to 210°C. The reaction product is crystallized and sliced to obtain the finished product guanidine nitrate. Nitric acid can also be produced through the interaction of calcium cyanamide and nitric acid. guanidine. ![]()
Purpose
1. Mainly used for the preparation of nitroguanidine, which is the basic raw material and intermediate for dozens of drugs such as sulfamidine, trimethoprim, and pyrimethamine.
2. Used in the manufacture of explosives, disinfectants, photographic chemicals, etc. [14]

 微信扫一扫打赏
微信扫一扫打赏

