Background and overview[1][2]
O-xylylenediamine dihydrochloride is the hydrochloride form of o-xylylenediamine and can be prepared from o-xylene through a two-step reaction.
Preparation[1]
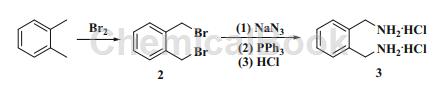
Apply[2]
The free base of o-xylylenediamine dihydrochloride was used to prepare a polyamide VOCs interception polymer separation membrane.
CN201710348456.0 provides a method for preparing a polyamide VOCs interception polymer separation membrane. The method includes the following steps:
Step 1: Using water as the solvent, compound A is oxidized to compound B under the catalysis of the phase transfer catalyst quaternary ammonium salt;
Step 2: The generated compound B and the compound monomer containing an amine group undergo a polymerization reaction under the action of an acid binding agent and the catalyst triphenyl phosphite. After the reaction, the polymer solution obtained is poured into deionized water. After sedimentation in water, suction filtration, and vacuum drying, the oligomer is obtained. The oligomer is dissolved in an organic polar solvent, and after ultrasonic breakage, a film liquid coating is obtained;
The third step: Coat the obtained membrane liquid on the solvent-resistant polysulfone bottom membrane, cross-link and dry it in a vacuum, and obtain a triptylenyl VOCs-retaining polymer separation membrane.
In the technical solution of the present invention: the compound monomer containing an amine group in the second step is selected from diamine compounds or tetraamine compounds;
The diamine compound is selected from 5,5′-diamino-2,2’bipyridine, 2,2′-bis(trifluoromethyl)-4, 4′-diaminophenyl Ether, 2,2-bis[4-(4-aminooxybenzene)]hexafluoropropane, 2,2′,5,5′-tetrachlorodiphenylamine, 2,3,5, 6-tetramethyl- 1,4-phenylenediamine, 3,3′,5,5′-tetramethylbenzidine, m-phenylenediamine, p-phenylenediamine, benzidine, ortho-toluidine, 3,3′-dimethoxy benzidine, 2,3,5,6-tetrafluoro-p-phenylenediamine, o-xylylenediamine, 5-chlorom-phenylenediamine, 1, 3-phenylenediamine, 4-methoxym-phenylenediamine Phenylenediamine, benzo[1,2-d;4,5-D]bisthiazole-1,6-diamine, 2-nitro-1,4-phenylenediamine, 2,5-di-chloro- 1,4-phenylenediamine, 4,4′-diaminobenzoanilide, toluene-2,5-diamine, 4-nitro-1,3-phenylenediamine, 2-chloro-4-nitro -1,3-phenylenediamine, oxybenzidine, tetrafluoroisophthalimide, pyromellitic acid diamine, 2,2,4, 4-tetramethyl-1,3-cyclobutane Diamine, triethylenetetramine, 4,6-diaminopyrimidine or 3,5-diamino-1,2,4-triazole.
Main reference materials
[1] Wang Z G , Yu W , Zheng H Q , et al. Synthesis and Crystal Structure of a Novel Glycoluril Molecular Scaffold[J]. Journal of Chemical Crystallography, 2016, 46(4):208-212.
[2] CN201710348456.0 A polyamide VOCs interception polymer separation membrane and its preparation method

 微信扫一扫打赏
微信扫一扫打赏

