Question: Is the oxygen hybridization of phenol SP3 or sp2? What are the number of atoms and the number of electrons involved in the large π bond formed by phenol?
View 1:
sp2, the number of electrons involved in forming a large π bond is 7.
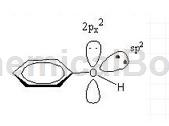
View 2:
The number of electrons involved in forming a large π bond is 8
Compounds in which the hydroxyl group is directly connected to the aromatic ring are called phenols, with the general formula ArOH. The oxygen atom of the phenolic hydroxyl group is in the sp2 hybridized state. There are two lone pairs of electrons on the oxygen. One pair occupies the sp2 hybrid orbit and the other pair occupies the p orbital that is not involved in hybridization. The p electron cloud can just match the large π of the benzene ring. The bond electron clouds overlap sideways to form a p-π conjugated system. Therefore, the delocalized bond in phenol is composed of 7 atoms (6 C atoms and 1 O atom on the benzene ring) sharing 8 electrons (6 C atoms on the benzene ring each provide 1, and the O atom provides a pair p electrons are 2 electrons) (as shown in the figure below).
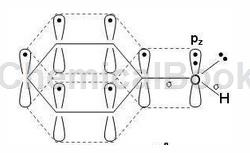
In the p-π conjugated system, the p electron cloud of oxygen transfers to the benzene ring, which reduces the density of the electron cloud on the oxygen atom, which weakens the binding force between O—H, allowing hydrogen to react as H+ The form dissociates and becomes acidic. In other words, the results of p-π conjugation are as follows: ① Enhanced dissociation ability of hydrogen on the hydroxyl group, ② Increased electron cloud density on the benzene ring. The specific properties are that phenol is weakly basic and is prone to electrophilic substitution on the aromatic ring (the ortho-para position of the hydroxyl group).

 微信扫一扫打赏
微信扫一扫打赏

