Background and overview[1][2][3]
The chemical formula of sodium sulfide is Na2S, also known as alkali sulfide, also known as stinky soda and stinky alkali. Colorless or slightly purple prismatic crystals. There is an eggy smell. The specific gravity of water is 2.427. The melting point is 52℃ and decomposes at 920℃. Soluble in water and show strong alkaline reaction. It is used as the main agent in the photo brown toning method, because sodium sulfide can react with silver salts bleached by potassium ferricyanide to produce tan silver sulfide. In addition, it can be used in blackening liquids thickened with copper bromide and silver nitrate or thickened with red blood salt and lead nitrate in wet plate photography in the printing industry.
Sodium sulfide usually exists in the form of nonahydrate Na2S·9H2O. Anhydrous and nonahydrate are both colorless and soluble solids, which become strongly alkaline when hydrolyzed in aqueous solution. When exposed to the air, sodium sulfide releases toxic hydrogen sulfide gas that smells like rotten eggs. The formula of sodium sulfide nonahydrate is 240.18. Colorless crystal, easily deliquescent, soluble in water and ethanol. After hydrolysis, the solution is alkaline and corrosive. When it comes into contact with skin or cutin, it can soften and dissolve it and cause hair to fall off.
Dissolved in crystal water at 50°C. It turns yellow when exposed to light and can be oxidized into sodium thiosulfate in the air. When sulfur is added to an aqueous solution, sodium polysulfide (Na2Sx, x=2, 3, 4 or 5) is generated. Used in the preparation of sulfur dyes, leather hair removers, and as reducing agents in the organic chemical industry. By mixing and calcining sodium sulfate with carbon, sodium sulfate can be reduced to form sodium sulfide. It should be stored in a ventilated, dry place, away from light, and the container should be sealed to prevent moisture and deterioration. Keep away from heat sources and living areas, and isolate from explosives, chemicals, oxidants, etc. Wear protective gear and rubber gloves when loading and unloading. Firefighting can use water or sand.
Apply[4][5]
Sodium sulfide nonahydrate can be used to manufacture dyes and sulfides, and is used as ore flotation agent, hide dehairing agent, paper cooking agent, etc. It is used as an analytical reagent and is often used as a precipitating agent for metal ions such as cadmium. Also used in photography, mineral flotation, metal processing, zinc and cadmium electroplating. Examples of its application are as follows:
1. Synthesis of thiophenols based on sodium sulfide nonahydrate. The specific steps are as follows: under the protection of inert gas, in an aprotic polar solvent, add substituted iodobenzene and sulfhydrylation reagent, then add copper salt catalyst and ligand compound in sequence, and react at 90-120°C for 12-24 hours. A reaction liquid is obtained. After the reaction liquid is cooled to room temperature, it is acidified to obtain the product. Its synthesis route is as follows:
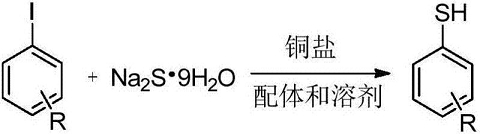
2. Preparation of trimethylsulfonium chloride. Trimethylsulfonium chloride is a trimethylsulfonium salt, which can be used as a methylation reagent for the methylation reaction of carboxylic acids and amine compounds, and can also be used in the preparation of corresponding epoxy compounds from aldehydes and ketones, especially It can be used as a pesticide intermediate in the production of herbicide glufosinate. The preparation method is to place sodium sulfide nonahydrate and methane chloride in a pressure reactor with alcohol as the solvent, pressurize and react for 4 to 10 hours at 65 to 100°C, then lower to room temperature and filter, and the filtrate will be synthesized after removing the solvent. trimethylsulfonium chloride.
The reaction equation of the present invention is as follows:
![]()
Preparation [4]
Method 1: To prepare sodium sulfide nonahydrate, dissolve 100g sodium hydroxide in 200g water, and then pass in pure H2S gas under cooling. When bubbles no longer occur, it reaches saturation. At this time, brown impurities precipitate out. Filter and immediately seal the container to isolate air. Leave it in a cool place for three days to precipitate crystals, filter, wash twice with a small amount of ethanol, and dry in a concentrated sulfuric acid desiccator to obtain sodium sulfide nonahydrate.
Method 2: Use industrial sodium sulfide nonahydrate as raw material for purification. Dissolve industrial sodium sulfide in hot water to make an aqueous solution containing 30% sodium sulfide. After filtration, the filtrate is cooled and crystallized with ice under strong stirring. After the crystallization is filtered, an appropriate amount of barium hydroxide solution is added, heated, filtered and cooled. After the obtained crystals are suction-filtered, dissolve them in water under heating conditions, add a small amount of zinc oxide, stir until the solution is clear, and then filter. The filtrate is cooled with ice and crystallized under constant stirring. After suction filtration, white nonahydrate is obtained.
Method 3: Pour hydrogen sulfide gas into the 20% sodium hydroxide solution until it is saturated, then add the same amount of 20% sodium hydroxide solution. Stir well and filter quickly. When the filtrate is heated and evaporated and concentrated to 1/2 of the original volume, add sodium sulfide nonahydrate seed crystals, cool the crystals with ice, filter the crystals quickly, and wash them 2 to 3 times with ethanol.
Main reference materials
[1] Concise Photography Dictionary
[2] Practical Chemistry Dictionary for Middle School Teachers
[3] Technical Dictionary of Container Transport Business·Volume 2
[4] Liu Yajun;
[5] Li Yan; Wu Zhongzhong; Guo Ting; Ren Jun; Zhang Wanxuan; Chen Zuxing. A method for preparing trimethylsulfonium chloride. CN201711333050.1, application date 20171213

 微信扫一扫打赏
微信扫一扫打赏

