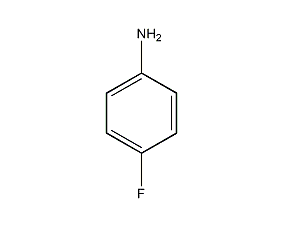
structural formula
| business number | 04hu |
|---|---|
| molecular formula | c6h6fn |
| molecular weight | 111.12 |
| label |
1-amino-4-fluorobenzene, 1-amino-4-fluorobenzene, aromatic nitrogen-containing compounds and their derivatives |
numbering system
cas number:371-40-4
mdl number:mfcd00007829
einecs number:206-735-5
rtecs number:by1575000
brn number:742030
pubchem number:24894858
physical property data
1. properties: light yellow oily liquid with pungent odor. [1]
2. melting point (℃): -1.9[2]
3. boiling point (℃): 188[3]
4. relative density (water = 1): 1.17[4]
5. octanol /water partition coefficient: 1.15[5]
6. flash point (℃): 73.9[6]
7 .solubility: slightly soluble in water, soluble in ethanol, ether, etc. [7]
toxicological data
1. acute toxicity[8] ld50: 417mg/kg (rat oral)
2. irritation[9]
rabbit transdermal: 2mg (24h), severe irritation.
rabbit eye: 250μg (24h), severe irritation.
ecological data
1. ecotoxicity no data available
2. biodegradability no data available
3 .non-biodegradability no information available
molecular structure data
1. molar refractive index: 30.48
2. molar volume (cm3/mol): 95.9
3. isotonic specific volume (90.2k ): 240.2
4. surface tension (dyne/cm): 39.3
5. polarizability (10-24cm3): 12.08
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 2
4. number of rotatable chemical bonds: 0
5. number of tautomers: none
6. topological molecule polar surface area 26
7. number of heavy atoms: 8
8. surface charge: 0
9. complexity: 66.9
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
1. stability[10] stability
2. incompatible substances[11] acids, acid chlorides, acid anhydrides, chloroform, strong oxidants
3. conditions to avoid contact[12] heat
4. polymerization hazard[13] no aggregation
5. analysis�product[14] hydrogen fluoride, ammonia
storage method
storage precautions[15] store in a cool, ventilated warehouse. keep away from fire and heat sources. keep container tightly sealed. they should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. equipped with the appropriate variety and quantity of fire equipment. the storage area should be equipped with emergency release equipment and suitable containment materials.
synthesis method
1. one-step method the better process among the one-step method is the nitrobenzene method, which is obtained by deoxygenation, hydrogenation and fluorination. using pdcl2-v2o5 as the catalyst, carbon monoxide as the reducing agent, and reacting at 160°c for 3 hours, the yield of 4-fluoroaniline can reach 90%, and 10% of the by-product aniline. using pto2 as the catalyst, bf3-hf as the fluorinating agent, and hydrogen as the reducing agent, the reaction was carried out for 12.5 hours at 42°c, with a reaction conversion rate of 100% and a yield of 95%.
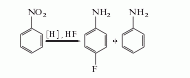
in addition, there are phenylhydroxylamine method and aniline method.
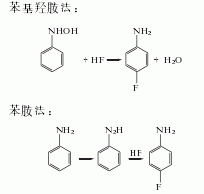
2. the two-step method differs from the raw materials are used to synthesize p-fluoronitrobenzene, and then reduced and hydrogenated to produce p-fluoroaniline. there are many methods: (1) p-chloronitrobenzene fluorination method the fluorination catalyst uses styrene copolymer as the matrix, grafted with tetraphenylphosphine or n-alkylaminopyridine salt, quaternary ammonium salts, crown ether and polyethylene glycol, etc. solvents used in fluorination reactions include dimethyl sulfoxide, dimethylformamide, dimethylacetamide and sulfolane.
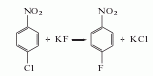
(2) nitrobenzene fluoride chemical methods include: electrolytic fluorination method uses an electrolyte composed of nitrobenzene, hydrogen fluoride and quaternary ammonium salt or et3n/hf, and passes a current of 3-5ma/cm2 at room temperature for electrolytic fluorination. use elemental fluorine or agf2 as the fluorinating agent to pass fluorine gas and nitrogen into the formic acid aqueous solution containing nitrobenzene to produce ortho-, meta-, and para-position mixed fluorinated nitrobenzene; use agf2 as the fluorinating agent, and reflux the nitrobenzene and chloroform solutions for 18 hours to obtain mixed fluorinated nitrobenzene.
3. reduction method of p-fluoronitrobenzene.
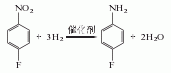
purpose
1. used to synthesize new fluorine-containing medicines, pesticides and dyes, etc.
2. used as an intermediate in the manufacture of herbicides and dyes. [16]

 微信扫一扫打赏
微信扫一扫打赏

