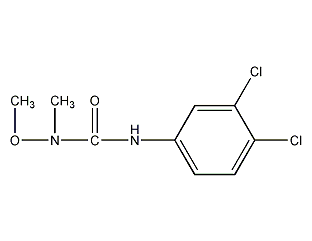
structural formula
| business number | 048w |
|---|---|
| molecular formula | c9h10cl2n2o2 |
| molecular weight | 248 |
| label |
afalon,aphalon,cephalon,lorex,lorox,sinuron, herbicide |
numbering system
cas number:330-55-2
mdl number:mfcd00055249
einecs number:200-659-6
rtecs number:ys9100000
brn number:2128725
pubchem number:24858583
physical property data
一 , physical property data
traits :white crystal
density (g/ml,25/4℃): not available
relative vapor density (g/ml, air=1):not available
melting point (ºc): 93-94
boiling point (ºc, normal pressure): 180-190
boiling point (ºc, 5.2kpa): not available
refractive index: not available
flash point (ºc): not available
optical rotation (º): not available
spontaneous combustion point or ignition temperature (ºc): not available
steam pressure (kpa, 25ºc): not available
saturated vapor pressure (kpa, 60ºc): not available
burn heat (kj/mol):not available
critical temperature (ºc): not available
critical pressure (kpa): not available
oil and water log value of the (octanol/water) partition coefficient:not available
explosion upper limit (%, v/v): not available
explosion lower limit (%, v/v): not available
dissolve properties:soluble in acetone and ethanol
toxicological data
two , toxicological data:
acute toxicity:ld50: 1500 mg/kg (rat oral); 2400 mg/kg (mouse oral) mouth) .
ecological data
three , ecological data:
1 ,other harmful effects: the substances are harmful to the environment, and special attention should be paid to water pollution.
molecular structure data
1. molar refractive index:60.41
2. molar volume (m3/mol):176.5
3. isotonic specific volume (90.2k): 467.8
4. surface tension (dyne/cm): 49.3
5, polarizability(10-24cm3): 23.95
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 2
4. number of rotatable chemical bonds: 2
5. number of tautomers: 2
6. topological molecule polar surface area 41.6
7. number of heavy atoms: 15
8. surface charge: 0
9. complexity: 228
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none
storage method
none
synthesis method
by3,4- dichlorophenyl isocyanate reacts with hydroxylamine sulfate to form3, 4-dichlorophenyl hydroxyurea, then with sulfuric acid dimethyl ester reaction produces linuron. 1.3,4-preparation of dichlorophenyl hydroxyurea will3,4 –dichlorophenyl isocyanate– toluene solution, hydroxylamine sulfate aqueous solution, and sodium hydroxide solution are pumped into each metering tank according to the calculated amount. add water and hydroxylamine sulfate into the glass-lined reaction pot at the same time, stir and cool, and stir at 20℃start adding sodium hydroxide solution dropwise from left to right, about1hafter dripping, control the temperature at20℃left and right, dripping is completephat7.5- 7.9 (otherwise add sodium hydroxide or hydroxylamine sulfate). then start adding drops3,4-dichlorophenyl isocyanate–toluene solution, control the temperature at this time30±2℃, about1.5hfinish the dripping and continue the reaction at this temperature2h. let sit2h, and then pump out the toluene clear liquid in the upper layer . add water to the lower material, stir it, and then centrifuge to obtain a solid wet product3, 4-dichlorophenyl hydroxyurea . yield87%. 2.preparation of liguron ; mso-ascii-font-family: ‘times new roman’; mso-hansi-font-family: ‘times new roman'”>add the above wet hydroxyurea into the glass-lined reaction kettle, and then��add the calculated amount of dimethyl sulfate and stir20min. add drops20%sodium hydroxide solution, temperature control within20-30℃, about1.5h has been added. in30℃left and right reactions2h, toph7 is the end point of the reaction. if it is acidic, sodium hydroxide can be added appropriately. after the reaction is completed, add water, stir, filter, and dry to obtain ligurong original powder. yield90%.
purpose
liguron can be used in corn, wheat, cotton, soybeans, sorghum, peanuts, peas, potatoes, upland rice, sunflowers, sugar cane, flax and a variety of vegetables control single and dicotyledonous weeds and certain perennial weeds in crop fields such as fruit trees and forest nurseries. oral administration to rats : 10pt; color: #333333; font-family: 宋体; mso-ascii-font-family: ‘times new roman’; mso-hansi-font-family: ‘times new roman'”>for4000mg/kg.

 微信扫一扫打赏
微信扫一扫打赏

