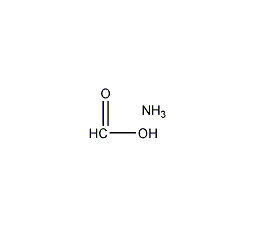
Structural formula
| Business number | 05KD |
|---|---|
| Molecular formula | CH5NO2 |
| Molecular weight | 63.06 |
| label |
ammonium formate, ammonium formate, Formic acid ammonium salt, Formic acid,ammonium salt, Analytical reagents, precipitating alkali metals |
Numbering system
CAS number:540-69-2
MDL number:MFCD00013103
EINECS number:208-753-9
RTECS number:BQ6650000
BRN number:3625095
PubChem ID:None
Physical property data
1. Properties: Colorless or white monoclinic crystal or powder.
2. Density (g/mL, 25/4℃): 1.266
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 119~121
5. Boiling point (ºC, normal pressure): 180
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor Pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, soluble in ethanol and ammonia .
Toxicological data
Acute toxicity: Mouse oral LC50: 2250mg/kg, no detailed description except lethal dose;
Mouse intravenous LC50: 410mg/kg, no detailed description except lethal dose;
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 41.1
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 7.5
10. Number of isotope atoms: 0
11. Determine the atomic configurationNumber of stereocenters: 0
12. Uncertain number of stereocenters of atoms: 0
13. Determined number of stereocenters of chemical bonds: 0
14. No Determine the number of stereocenters of chemical bonds: 0
15. The number of covalent bond units: 2
Properties and stability
1. Use and store according to specifications. It will not decompose and avoid contact with oxides.
2. It has a pungent smell and is easy to deliquesce. Easily soluble in water and ethanol. At 0°C, 102g of ammonium formate can be dissolved in 100g of water; at 20°C, 143g of ammonium formate can be dissolved in 100g of water; at 80°C, 531g of ammonium formate can be dissolved in 100g of water.
Storage method
This product should be kept sealed and dry.
Synthesis method
1. Obtained from the neutralization of formic acid and ammonia. Freeze formic acid and water, circulate ammonia gas under stirring to make the pH value 7-7.5, cool to -5°C, and filter to obtain ammonium formate.
![]()
2. In 90% formic acid solution in, introduce ammonia gas.
After passing in a sufficient amount of ammonia gas, the temperature rises, and the heat released can promote the precipitation of ammonium formate. Filter out the crystals, recrystallize them with absolute ethanol, and dry them in a vacuum desiccator with 99% sulfuric acid.
3. 100 parts of methyl formate, 100 parts of water, 0.1 part of formic acid, adjust the pH value to 2.5, heat to 70°C with stirring, add 20 parts of ammonia water, keep the pH value at 2 to 3, After about 1 hour, 70% of methyl formate has been hydrolyzed. Internal steaming is performed. When the distillation head temperature is 10°C, unreacted methyl formate and methanol are evaporated, leaving an acidic ammonium formate solution in the evaporator. Neutralize with ammonia and then evaporate to obtain ammonium formate with a content of 98%.
Purpose
1. Analytical reagents. Precipitation of alkali metals from noble metal salts.

 微信扫一扫打赏
微信扫一扫打赏

