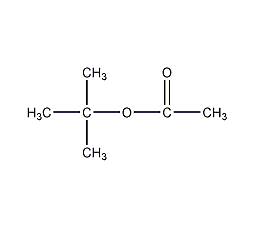
Structural formula
| Business number | 05KH |
|---|---|
| Molecular formula | C6H12O2 |
| Molecular weight | 116.16 |
| label |
tert-butyl acetate, 1,1-dimethylethyl acetate, tert-butyl acetate, Terbutyl acetate, tert-butyl acetate, tert-butyl acetate, Acetic acid 1,1-dimethylethyl ester, CH3COOC(CH3)3, gasoline additives |
Numbering system
CAS number:540-88-5
MDL number:MFCD00008807
EINECS number:208-760-7
RTECS number:AF7400000
BRN number:1699506
PubChem ID:None
Physical property data
1. Properties: colorless liquid with fruity aroma. [1]
2. Melting point (℃): -77.9[2]
3. Boiling point (℃): 96[3]
4. Relative density (water=1): 0.86 (25℃)[4]
5. Relative vapor density (air = 1): 4[5]
6. Saturated vapor pressure (kPa): 6.3 (25℃)[6]
7. Critical pressure (MPa): 3.17[7]
8. Octanol/water partition coefficient: 1.76[ 8]
9. Flash point (℃): 16.6~22.2 (CC) [9]
10. Ignition temperature ( ℃): 421[10]
11. Explosion upper limit (%): 7.3[11]
12. Explosion Lower limit (%): 1.3[12]
13. Solubility: Insoluble in water, soluble in most organic solvents such as ethanol, ether, and acetic acid. [13]
14. Liquid phase standard hot melt (J·mol-1·K-1): 236.8
15. Solubility parameter (J·cm-3)0.5: 16.040
16. van der Waals area (cm2·mol-1): 1.070×1010
17. van der Waals volume (cm3·mol-1): 73.200
Toxicological data
1. Acute toxicity[14]
LD50: 4100mg/kg (rat oral); >2g/kg (rabbit oral Skin)
LC50: >2230mg/m3 (rat inhalation, 4h)
2. Irritation[15]
Rabbit transdermal: 500μl (24h), mild stimulation.
Rabbit eye: 100μl, mild irritation.
Ecological data
1. Ecotoxicity[16] IC50: 420mg/L (72h) (algae)
2. Biodegradability No data yet
3. Non-biodegradability[17] In the air, when the hydroxyl radical concentration is 5.00×105 pieces/cm3, the degradation half-life is 29 days (theoretical).
4. Other harmful effects [18] This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 31.58
2. Molar volume (cm3/mol): 131.1
3. Isotonic specific volume (90.2K ): 290.6
4. Surface tension (dyne/cm): 24.1
5. Polarizability (10-24cm3): 12.52
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 89.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[19] Stable
2. Incompatible substances[20] Strong oxidants, strong acids, strong alkali
3. Polymerization hazard[21] No polymerization
Storage method
Storage Precautions[22] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. Keep container tightly sealed. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Acetic anhydride method: Mix tert-butyl alcohol, acetic anhydride and anhydrous zinc chloride, heat and reflux for 2 hours, then cool, distill until the temperature reaches 110°C, wash the crude distillate with water, and then use 10% potassium carbonate Wash until neutral, dry with anhydrous potassium carbonate, and then distill. Collect the 95-96°C fraction to obtain the finished product.
2. Acetyl chloride method: Mix tert-butanol; N,N-dimethylaniline and anhydrous ether, heat to reflux, add acetyl chloride, control the feeding temperature, maintain a medium reflux speed, and add When 2/3 acetyl chloride is used, N,N-dimethylaniline hydrochloride is crystallized, cooled in an ice bath, and then heated for 1 hour. Add water to completely dissolve the solid matter, separate the ether layer, extract with cold 10% sulfuric acid, wash once with saturated sodium bicarbonate solution, dry with anhydrous sodium sulfate, and fractionate to obtain the finished product.
3. Acetyl chloride-magnesium method: Mix magnesium powder, tert-butyl alcohol and anhydrous ether, dropwise add acetyl chloride and anhydrous ether solution, cool after adding, add cold potassium carbonate aqueous solution, make Decompose it, extract it with ether three times, dry it with calcium chloride, and distill it to get the finished product.
4. Preparation method:
In a reaction bottle equipped with a stirrer, dropping funnel, and reflux condenser, add 57g (0.77mol) of dry tert-butanol (2) ), N, N-dimethylaniline 101g (106mL, 0.84mol), anhydrous ether 100mL. Heat to reflux with stirring. Add 63g (0.80mol) of acetyl chloride dropwise, and control the dropping speed until the heat source is removed and the reflux state is maintained. When about 2/3 of the acetyl chloride is added, the ammonium salt precipitates and the reaction proceeds violently, and it can be cooled with an ice-water bath. Slowly add the remaining acetyl chloride. After the addition, continue the reflux reaction for 1 hour. Cool to room temperature, add 100 mL of water, and stir thoroughly to completely dissolve the solid matter. Separate the organic layer. The organic layer was washed with 10% sulfuric acid, then with saturated sodium bicarbonate solution, and dried over anhydrous sodium sulfate. The ether was distilled off, fractionated, and the fractions at 96-98°C were collected to obtain 54g of tert-butyl acetate (1) with a yield of 61%. [24]
Purpose
Used as a solvent for digesting cellulose, etc. and as a gasoline additive. [23]

 微信扫一扫打赏
微信扫一扫打赏

