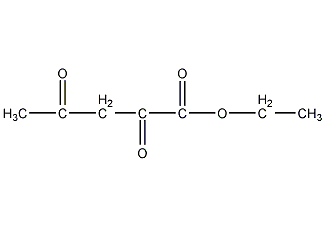
Structural formula
| Business number | 06GQ |
|---|---|
| Molecular formula | C7H10O4 |
| Molecular weight | 158.15 |
| label |
Ethyldiazole ester, Ethyl acetylacetonate, Acetone ethyl oxalate, Acetylacetonate, Ethyl acetonoxalate, CH3COCH2COCOOC2H5 |
Numbering system
CAS number:615-79-2
MDL number:MFCD00009124
EINECS number:210-447-5
RTECS number:None
BRN number:607062
PubChem number:24853912
Physical property data
1. Physical property data
1. Melting point (ºC): 16-18ºC
2. Boiling point (ºC, normal pressure): 101-103 °C12 mm Hg(lit .)
3. Density: 1.126 g/mL at 25 °C(lit.
4. Refractive index: n20/D 1.474(lit .)
5. Flash point (ºC): 230 °F
Toxicological data
None
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 36.31
2. Molar volume (cm3/mol): 141.8
3. Isotonic specific volume (90.2K): 345.5
4. Surface tension (dyne/cm): 35.2
5. Polarizability (10-24cm3): 14.39
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.2
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: 5
6. Topological molecule polar surface area 60.4
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 183
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Properties and stability:
The product may not decompose under normal temperature and pressure.
Storage method
Storage:
Seal the container and store it in a sealed main container in a cool, dry place.
Synthesis method
1. Production method: Obtained from the condensation of oxalic acid diester and acetone (Kirschner reaction). Add sodium methoxide dropwise into diethyl oxalate and acetone at 40-45°C, keep warm for 1 hour, and add concentrated sulfuric acid dropwise to pH=3.5 while cooling. Extract with benzeneThe reactants and extracts were distilled under reduced pressure after benzene was removed. Collect the 130-132°C (4.93kPa) fraction to obtain the product ethyl acetylacetonate. 2. Preparation method: 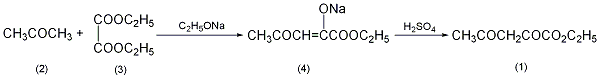 In a reaction flask equipped with a stirrer, reflux condenser and dropping funnel , add 2L of absolute ethanol, and add 63g (2.7mol) of metallic sodium in batches while stirring. After all the sodium has reacted, cool to room temperature. Add dropwise a mixture of 365g (2.5mol) of diethyl oxalate (3) and 145g (2.5mol) of acetone (2), and finish adding ① in about 3 hours. After the addition is completed, raise the temperature to 40°C and continue stirring for 1 hour to form a yellow viscous substance. Cool, filter with suction, and wash with ethanol to obtain sodium salt (4) ②. Add the sodium salt to 1.3g of ice water, slowly add it to the pre-frozen dilute sulfuric acid (prepared with 100mL concentrated sulfuric acid and 200mL water) while stirring, control the temperature of the reaction solution at 0~5°C, adjust to pH 3~4, and continue stirring for 10 minutes , until the yellow sodium salt disappears. Extract with benzene (300mL×3), combine the extracts, and wash with water. Benzene was recovered by distillation under normal pressure, and then distilled under reduced pressure to collect the fraction at 117~121℃/308kPa to obtain 240g of ethyl acetylacetonate (1)③, with a yield of 61%. Note: ① A white precipitate is formed in the early stage of the reaction, then gradually turns yellow, further deepens, and finally turns yellow, and the reaction system gradually thickens. ②Suction filtration is very slow, and finally centrifugation is used. ③The product has poor stability and will decompose on its own during storage. [1]
In a reaction flask equipped with a stirrer, reflux condenser and dropping funnel , add 2L of absolute ethanol, and add 63g (2.7mol) of metallic sodium in batches while stirring. After all the sodium has reacted, cool to room temperature. Add dropwise a mixture of 365g (2.5mol) of diethyl oxalate (3) and 145g (2.5mol) of acetone (2), and finish adding ① in about 3 hours. After the addition is completed, raise the temperature to 40°C and continue stirring for 1 hour to form a yellow viscous substance. Cool, filter with suction, and wash with ethanol to obtain sodium salt (4) ②. Add the sodium salt to 1.3g of ice water, slowly add it to the pre-frozen dilute sulfuric acid (prepared with 100mL concentrated sulfuric acid and 200mL water) while stirring, control the temperature of the reaction solution at 0~5°C, adjust to pH 3~4, and continue stirring for 10 minutes , until the yellow sodium salt disappears. Extract with benzene (300mL×3), combine the extracts, and wash with water. Benzene was recovered by distillation under normal pressure, and then distilled under reduced pressure to collect the fraction at 117~121℃/308kPa to obtain 240g of ethyl acetylacetonate (1)③, with a yield of 61%. Note: ① A white precipitate is formed in the early stage of the reaction, then gradually turns yellow, further deepens, and finally turns yellow, and the reaction system gradually thickens. ②Suction filtration is very slow, and finally centrifugation is used. ③The product has poor stability and will decompose on its own during storage. [1]
Purpose
Usage: Intermediate of pharmaceutical sulfonamides.

 微信扫一扫打赏
微信扫一扫打赏

