Background
Parachlorostyrene, also called 4-chlorostyrene, p-chlorostyrene (referred to as PCST), is an intermediate in organic synthesis and pharmaceutical research and development, and can be used to make plastics and rubber.
Structure
![]()
Purpose[1]
It is used as reaction intermediate and main lipid modifier. Its derivatives are widely used, such as p-divinylbenzene, p-styrene diphenylphosphine, and p-vinyl benzoic acid. These derivatives are used in ion exchange resins, Used in functional polymers, photosensitive polymers, polymer catalysts, medicines, and pesticides.
Preparation[2][3][4][5]
Using Grignard coupling method to prepare high-purity p-chlorostyrene and its derivative monomers:
Japan’s Hokko Chemical Company has developed a technology to synthesize p-chlorostyrene (referred to as PCST) using p-dichlorobenzene as a starting material and a Grignard coupling reaction (Grinard coupling reaction), as well as p-chlorostyrene derived from PCST. Three monomers, including divinylbenzene (PDVB), p-vinylphenyl diphenylbenzene (PSDP), and p-vinyl benzoic acid (PVBA), have entered the market for development. PCST prepared by the company’s method is different from Different from other methods, the position of the substituent is limited to the para-position of high-purity products, so the derivative monomers are also para-position. Therefore, the physical properties of polymers prepared from these monomers are almost unknown, and it is expected to be discovered. New functions and properties.
The Grignard reaction is a reaction using the Grignard reagent RMgX, which is obtained by reacting organic halides and metal magnesium in anhydrous ether. It can synthesize alcohols, ketones, aldehydes, carboxylic acids, sulfides and other organic compounds. , and can also produce compounds that are difficult to obtain by other methods. It is said that the company uses tetrahydropyran as a solvent, thereby expanding industrial applications and extensively developing organotin compounds, organophosphorus compounds, organoboron compounds, medicines, and fragrances and other methanol (carbinol) compounds used as intermediates, and research on coupling reaction products. The company began to develop this new synthesis reaction in 1981. Using this reaction, p-dichlorobenzene and vinyl chloride were used The reaction formula for preparing p-chlorostyrene (PCS) T as raw material is as follows
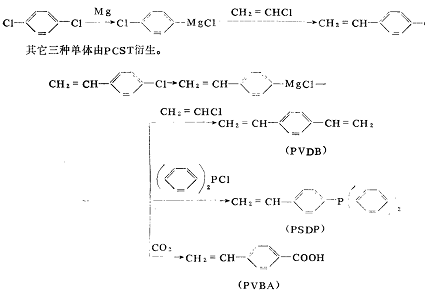
But the polymerization method of monomers is simpler than the polymer reaction, and what is obtained is a polymer with a clear structure. Designing these monomers to copolymerize with other monomers or monomers with other functional groups to produce new polymers. It is possible. Compared with styrene, PSCT has a polymerization speed more than twice that of styrene. If it is used to cross-link styrene of unsaturated polyester, the curing speed can be increased. It can also be used for the same purposes as styrene and can improve the flammability. and other physical properties.
Divinylbenzene, as a cross-linking monomer, can be used as a modifier in the manufacture of ion exchange resins and other resins. However, in the past, commercially available products were mixtures of meta- and para-mers, which were produced by the Grignard method. The obtained PDVB is completely para-mer, so it has the advantage of easily adjusting the cross-linking density and cross-linking structure. In addition, the copolymer of PDVB and styrene is expected to be used as an insoluble functional carrier, or as a reaction utilizing its residual vinyl group sexUse compound. The polymer of PSDP becomes a polymer ligand of organic metals (nails, germanium, palladium, etc.) and can be used for the synthesis of polymer catalysts; at the same time, because it has a trivalent phosphine structure, it can be used as a wittigreaetion. Attractive functional polymers such as halides and reducing agents.
The polymer of PVBA is a raw material that uses its various reactions as an aromatic carboxylic acid and its carboxyl group to derive various functional polymers. In addition, if used as a copolymer component, it can impart acidity and hydrophilicity to the polymer. 、 Ion exchange and other properties, so it is expected to be used as a resin modifier.
References
[1] Long Qidong, Lei Xinghan. Application of conjugated nitroalkenes as synthons[J]. Chinese Medical Journal. 199 1, 22(3): 82 ~ 90.
[2] Liu Yunshan, Bei Xunzhi, Zhu Huiqin, etc. Application of nitroolefin compounds in organic synthesis[J]. Chemical Bulletin. 1993, 4: 6 ~ 12.
[3] Wang Xiuran, Wan Yubao. Synthesis of p-hydroxy-monitrostyrene [J]. Journal of Anqing Normal University (Natural Science Edition), 1999, 5(4): 64-65.
[4] Wang Ye, Yang Xiaosheng, Zheng Anpiao, etc. Synthesis of trans-9-arylnitroethene compounds[J]. Chemical Bulletin, 2002, 89: 557-559.
[5] Frederick A, Luzzio. The Henry. reaction:recent exam ples[J]. Tetrahedron, 2001, 57:915~945.

 微信扫一扫打赏
微信扫一扫打赏

