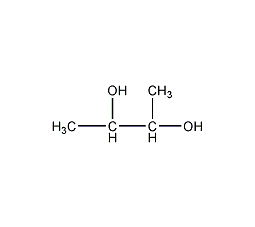
Structural formula
| Business number | 05B0 |
|---|---|
| Molecular formula | C4H10O2 |
| Molecular weight | 90.12 |
| label |
2,3-Dihydroxybutane, butanediol, 2,3-Butylene glycol, β,γ-Butylene glycol, 2,3-Dihydroxybutane, humidifier, plasticizer, coupling agent, alcohol solvent |
Numbering system
CAS number:513-85-9
MDL number:MFCD00004523
EINECS number:208-173-6
RTECS number:EK0532000
BRN number:969165
PubChem number:24851392
Physical property data
1. Properties: Colorless viscous liquid, crystals at low temperatures.
2. Boiling point (ºC, 2.13kPa): 89 (meso)
3. Boiling point (ºC, 98.9kPa): 181.7 (meso)
p>
4. Boiling point (ºC, 2.13kPa): 86 (racemate)
5. Boiling point (ºC, 98.9kPa): 176.7 (racemate)
6. Boiling point (ºC, 101.3kPa): 229.2(+)
7. Boiling point (ºC): 183~184
8. Melting point (ºC): 34.2~ 34.4 (meso)
9. Melting point (ºC): 25
10. Relative density (g/mL, 25/4ºC): 0.9939 (meso)
11. Relative density (g/mL, 25/4ºC): 0.9872(+)
12. Relative density (g/mL, 25/4ºC): 0.9869(-)
13. Refractive index (35ºC): 1.4324 (meso)
14. Refractive index (25ºC): 1.4310 (racemate)
15. Refractive index (25ºC): 1.4306(+)
16. Refractive index (25ºC): 1.4308(-)
17. Viscosity (mPa·s, 25ºC): 121(-)
18. Viscosity (mPa·s, 35ºC): 90(-)
19. Solubility: Miscible with water, soluble in alcohol and ether.
20. Melting point (ºC): 19
21. Boiling point (ºC): 180.7
22. Relative density (20℃, 4℃): 0.995
23. The refractive index at room temperature (n20): 1.433
24. The refractive index at room temperature (n25): 1.432
25. Solubility parameter (J·cm-3)0.5: 25.950
26. van der Waals area ( cm2·mol-1): 8.300×109
27. van der Waals volume (cm3·mol-1): 56.980
28. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -2520.9
29. Gas phase standard claimed heat (enthalpy) (kJ·mol– 1): -482.3
30. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2461.7
31. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -541.5
32. Liquid phase standard heat melt (J·mol-1 ·K-1): 221.7
33. Flash point (ºC): 85
Toxicological data
Acute toxicity: Mouse intraperitoneal LD50: 6075mg/kg, no details except lethal dose; Mouse oral LD50: 9080mg/kg
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 23.56
2. Molar volume (cm3/mol): 90.3
3. Isotonic specific volume (90.2K ): 219.5
4. Surface tension (dyne/cm): 34.8
5. Polarizability (10-24cm3): 9.34
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 30.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 2
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Use and store according to specifications. It will not decompose and avoid contact with oxides. Flammable liquids. It is hygroscopic and does not corrode metal.
2. Avoid inhalation; avoid contact with eyes, skin and clothing. It is hygroscopic and sensitive to air. Store in a dry and cool place under nitrogen protection.
Storage method
This product should be sealed and stored in a cool, dry place. Store in a cool, dry, well-ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from acids and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. It is produced by biological fermentation using sugar, molasses, malt slurry or alcohol mother liquor as raw materials. When sugar is fermented to produce alcohol and then vinegar, the by-product acetaldehyde can also be used to produce 2,3-butanediol under the action of yeast.
2. 2,3-Butanediol uses sulfuric acid as a catalyst and reacts with acetic acid at 140-150°C for 2 hours to generate 2,3-butanediol diacetate, which can be added to cream. Can be used as hygroscopic agent, plasticizer and coupling agent. 2,3-Butanediol is also used as a solvent and a raw material for synthetic resins.
3. Tobacco: FC, 9.
Purpose
1. 2,3-butanediol uses sulfuric acid as a catalyst and reacts with acetic acid at 140-150°C for 2 hours to generate 2,3-butanediol diacetate, which can be added to cream or used as a hygroscopic agent. , plasticizers and coupling agents. 2,3-Butanediol is also used as a solvent and a raw material for synthetic resins.

 微信扫一扫打赏
微信扫一扫打赏

