[Background and Overview][1][2][3]
Plasticizer, also known as plasticizer, is a polymer material additive that can be added to plastics to increase its plasticity, expansion or flexibility. As a cold-resistant plasticizer with excellent performance, dioctyl adipate has attracted more and more attention from the processing industry and the plastics industry in recent years. Dioctyl adipate (DOA) is a cold-resistant plasticizer for polyvinyl chloride and has certain heat resistance, light resistance and water resistance. Its toxicity is very low, and its oral LD50 for rats is 3 000~6 000 mg/kg, has little irritation to skin and eyes, and is also an important chemical intermediate. The synthesis of traditional DOA usually uses adipic acid and 2-ethylhexanol as raw materials, and uses concentrated sulfuric acid as a catalyst to perform an esterification reaction. However, it can only be produced by refining processes such as activated carbon decolorization, alkali neutralization, water washing, and dealcoholization. The product loss and equipment corrosion are serious, and a large amount of waste residue and wastewater pollution are produced. In view of the various drawbacks of concentrated sulfuric acid catalysis, the development of new high-quality, efficient, less corrosive, easy to separate, reusable, and low-cost catalysts has become an important topic in DOA catalytic synthesis, and this is also an inevitable trend in the development of today’s catalysts.
Dioctyl adipate is generally synthesized under acid catalysis using adipic acid and ethylhexanol isooctyl alcohol as raw materials. There are many kinds of catalysts currently used to synthesize dioctyl adipate, which can be summarized into the following categories: inorganic acid catalysts, organic acid catalysts, titanate catalysts, and solid acid catalysts. Among them, solid acid catalysts have the advantages of high catalytic activity, high selectivity, high temperature resistance, simple preparation, low pollution, and simple post-processing. They are an environmentally friendly catalyst with wide application prospects and have attracted much attention and attention. Therefore, using solid acid catalysts instead of other types of catalysts to catalytically synthesize dioctyl adipate has become a development trend in modern industry.
【Synthesis】[1][4]
There are many types of catalysts currently used to synthesize dioctyl adipate, which can be summarized into the following categories: inorganic acid catalysts, organic acid catalysts, titanate catalysts, and solid acid catalysts .
1. Organic acids
P-Toluenesulfonic acid is a strong organic acid and an industrial production catalyst that can replace concentrated sulfuric acid as a catalyst for DOA synthesis. Using p-toluenesulfonic acid as a catalyst is much less corrosive to equipment and producing “three wastes” than concentrated sulfuric acid as a catalyst, and has good activity, good selectivity, low price, low dosage, and is less likely to cause side reactions. , the product has good color, is easy to store, transport and use, and is very suitable for industrial production. Using microwave technology to synthesize DOA takes a short time, is easy to operate, saves energy, and has potential industrial application prospects.
2. Solid super acid
Since the 1940s, people have been looking for solid acids that can replace liquid acids, and solid super acids have become a hot research topic. Research shows that solid super acid is an acid with stronger acid strength than 100% sulfuric acid. It overcomes the shortcomings of liquid acid and has the characteristics of easy separation from the liquid phase reaction system, no corrosion of equipment, simple post-processing, little environmental pollution, and high selectivity. Characteristics, it can be used in a higher temperature range, expanding the application range of thermodynamically possible acid-catalyzed reactions. SO4 2- /TiO2-Al2O3-SnO2The surface of the catalyst has a certain degree of crystalline structure, which gives it good catalytic stability and resistance to coking. The use of this catalyst is not only easy to obtain raw materials, low in price, high in catalytic efficiency, and good in reusability, but more importantly, it has industrial application value.
3. Activated carbon fiber
Activity is a high-specific surface active material developed in the late 1970s. As a catalyst carrier, it not only helps to disperse active components, improves the effect of active phases, but also reduces the possibility of deactivation due to high-temperature sintering. Traditional activated carbon is a spark-treated porous carbon in powder or granular form, while activated carbon fibers are fiber piles. The fibers are covered with micropores. Their adsorption capacity for organic gases is several times higher than that of granular activated carbon in the air. Dozens of times, 5 to 6 times higher in aqueous solution, and the adsorption rate is 100 to 1000 times faster.
4.Heteropolyacid
Heteropoly acids are multi-protic acids with uniform strength. They have high catalytic activity and selectivity for many reactions, and have the advantages of non-volatility, good thermal stability, and fast regeneration speed. They are an ideal catalyst. It has been reported that heteropoly acids and their solid-supported catalysts are used to catalyze the reaction of adipic acid and n-octanol to synthesize DOA. The optimal reaction conditions are the molar ratio of acid to alcohol 1:2.4, and the amount of catalyst is 1.0% of the mass of adipic acid. 4%, reaction time 3.5h, esterification rate up to 99.1%. It has also been reported that di-n-octyl adipate was synthesized using adipic acid and n-octanol as raw materials, environmentally friendly phosphotungstic heteropoly acid as a catalyst, and toluene as a water-carrying agent. The optimal process conditions are: alkyd-to-acid molar ratio 2.8:1, the catalyst dosage is 1.2% of the acid mass, the water-carrying agent dosage is 75% of the acid mass, the reaction time is 3 hours, and the esterification rate reaches 99.23%.
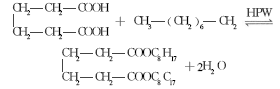
5. Molecular sieve
The concept of molecular sieve was proposed in 1932, which is an aluminosilicate poly.Porous crystal. With the in-depth research on molecular sieves, they have been widely used and occupy an important position. For example, petroleum processing, petrochemicals, fine chemicals and daily chemicals, etc. Utilizing the advantages of molecular sieves such as high adsorption capacity and strong thermal stability that other adsorbents do not have, adipic acid and 2-ethylhexanol are used as raw materials, and HY molecular sieve is used as a catalyst to synthesize DOA. The optimal reaction conditions are: reaction The temperature is 160°C, the adipic acid is 0.03 mol, the acid-to-alcohol molar ratio is 1:3, the catalyst dosage is 1.0 g, the reaction time is 5 hours, and the esterification rate can reach up to 98.7%.
[Application][1][2]
Dioctyl adipate is a cold-resistant plasticizer for polyvinyl chloride. It is commonly used in the processing of plastic and rubber products and as a plasticizer for synthetic resins. It is also used with main plasticizers such as DOP for cold-resistant agricultural films. , wires, sheets, artificial leather and packaging films for frozen foods. It can also be used as a low-temperature plasticizer for many synthetic rubbers and a plasticizer for resins such as nitrocellulose. According to the Toxicity of Chemical Substances and Environmental Protection Manual, it is a slightly toxic substance and can be used in food plastic wrap. It is also an excellent cold-resistant plasticizer for vinyl chloride copolymer, polyvinyl chloride, polystyrene, etc. It has a good plasticizing effect and can give products good light resistance and low-temperature flexibility. It is widely used in agricultural films.
[Main reference materials]
[1] Yin Xiuyun; Li Gan. Research on the synthesis of dioctyl adipate. Guangzhou Chemical Industry, 2014, 42.13: 24-26.
[2] Zou Yanhong. Research on the synthesis of dioctyl adipate catalyzed by solid acids. 2012. PhD Thesis. Changsha: Master’s Thesis of Hunan Normal University.
[3] Meng Qi, et al. Clean synthesis process of dioctyl adipate. Fine Petrochemicals, 2007, 24.2: 27-29.
[4] Luan Xianghai, et al. Catalytic synthesis and reaction kinetics of di-n-octyl adipate. Fine Chemicals, 2008, 25.12: 1232-1235.

 微信扫一扫打赏
微信扫一扫打赏

