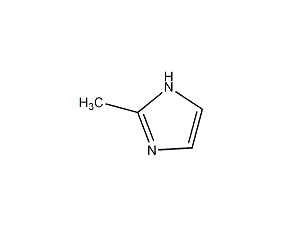
Structural formula
| Business number | 07A6 |
|---|---|
| Molecular formula | C4H6N2 |
| Molecular weight | 82.10 |
| label |
2-Methyl-1,3-azocene, 2-Methyl-1H-imidazole, 2-methylimidazole, 2-Methylglyoxaline, 2-methylimidazole, 2-Methyl-1H-Imidazole, 2-methylglyoxaline, Hardener, curing accelerator, Intermediates between the drug metronidazole and the feed growth promoter dimethazole |
Numbering system
CAS number:693-98-1
MDL number:MFCD00005190
EINECS number:211-765-7
RTECS number:NI7175000
BRN number:1368
PubChem number:24896976
Physical property data
1. Properties: white, beige or light yellow columnar crystals
2. Melting point (℃): 136 (142~143)
3. Boiling point (℃): 267
4. Solubility: Soluble in water, ethanol, slightly soluble in cold benzene.
Toxicological data
Acute toxicity: mouse (oral) LD50: 1400 mg/kg; rat (peritoneal) LD50: 480 mg/kg
Since the LD50 of table salt is 3,000 mg/kg, the acute toxicity of BPA The same level as table salt.
Can cause sensitizing reactions to the skin.
Ecological data
Do not allow undiluted or large quantities of products that are slightly hazardous to water to come into contact with groundwater, waterways or sewage systems. Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 23.60
2. Molar volume (cm3/mol): 77.2
3. Isotonic specific volume (90.2K): 198.6
4. Surface tension (dyne/cm): 43.7
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 9.35
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 28.7
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 44.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stay away from oxides and acids. This product is poisonous! Its toxicity is similar to that of diamines�. Production equipment must be sealed to prevent running, popping, dripping and leaking. Operators should wear protective equipment to avoid direct contact with this product.
2.Toxic, causing allergic reactions to the skin, and its toxicity is similar to diamines. Mice received LD501400mg/kg orally and LD50480mg/kg intraperitoneally. Operators should wear protective masks and rubber gloves.
Storage method
1. Store in a sealed container and keep in a cool, dry place. The storage area must be locked and the keys must be given to the technical experts and their assistants. Store away from oxidizing agents. Store in a cool, ventilated, dry place. Heat, moisture and sun protection. Store and transport according to regulations on toxic substances. 2. Packed in iron barrels or wooden barrels, lined with plastic bags and stored in a cool and ventilated place. Protect from heat, sun and moisture. Store and transport according to regulations on toxic chemicals.
Synthesis method
1. Obtained from 2-methylimidazoline by eliminating dehydrogenation. Heat and melt 2-methylimidazoline (melting point 107°C), carefully add active nickel, raise the temperature to 200-210°C and react for 2 hours. Lower the temperature to below 150°C, add water to dissolve, filter while hot, separate the active nickel, concentrate the filtrate until the temperature is above 140°C, discharge and cool to obtain 2-methylimidazole. Using this method to produce products with a purity of ≥98%, 1 ton of product consumes 1095kg of ethylenediamine (95%) and 975kg of acetonitrile. A better method is to use glyoxal and aldehydes as raw materials.
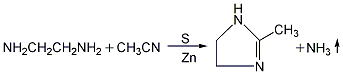
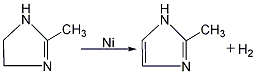
2.Heat and melt 2-methylimidazoline (melting point 107℃), carefully add active nickel, raise the temperature to 200~210℃, and catalyze dehydrogenation reaction for 2 hours. Lower the temperature to below 150°C, add water to dissolve, filter while hot, separate the active nickel, concentrate the filtrate until the temperature is above 140°C, discharge and cool to obtain 2-methylimidazole.
3.The glyoxal method uses glyoxal as raw material and reacts with acetaldehyde at around 45℃ Cyclization reaction with ammonia, then dehydration, crystallization and separation to prepare 2-methylimidazole. The advantages of the glyoxal process route are mainly the wide source of raw materials, short process route, low production cost, and low pollution. Therefore, this process has relatively strong advantages. All existing domestic manufacturers producing 2-methylimidazole adopt this process route.
Purpose
1. This product is an intermediate between the drug metronidazole and the feed growth promoter dimethazole. It is also a curing agent for epoxy resin and other resins. As a medium-temperature curing agent for epoxy resin, it can be used alone, but it is mainly used as a curing accelerator for powder molding and powder coating.
2. This product is also an intermediate of the drug metronidazole.

 微信扫一扫打赏
微信扫一扫打赏

