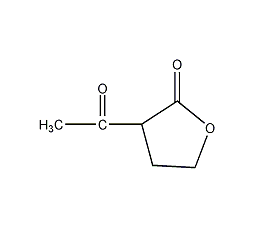
Structural formula
| Business number | 05BU |
|---|---|
| Molecular formula | C6H8O3 |
| Molecular weight | 128.13 |
| label |
2-acetobutyrolactone, 2-Acetyl-γ-butyrolactone, 3-Acetyldihydro-2((3H)-furanone, 3-Acetyldihydro-2(3H)-furanone, a-Acetylbutyrolactone, α-Acetobutyrolactone, Ester cyclic compounds and their derivatives |
Numbering system
CAS number:517-23-7
MDL number:MFCD00005394
EINECS number:208-235-2
RTECS number:None
BRN number:112676
PubChem number:24845124
Physical property data
1. Properties: liquid with ester-like odor.
2. Density (g/mL, 25/4℃): 1.1846
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): -12 to -13℃
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 3.99kPa ): 107~108 (666.5pa)
7. Refractive index (n20): 1.4562
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC) : Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13 . Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper limit of explosion (%, V/V): Undetermined
18. Explosion Lower limit (%, V/V): Undetermined
19. Solubility: Solubility in water is 20% (v/v).
Toxicological data
None
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 29.38
2. Molar volume (cm3/mol): 107.5
3. Isotonic specific volume (90.2K ): 269.3
4. Surface tension (dyne/cm): 39.3
5. Polarizability (10-24cm3): 11.65
Computing chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 5
6. Topological molecule polar surface area 43.4
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 150
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Use and store according to specifications. It will not decompose and avoid contact with oxides
2.This product has certain toxicity and is harmful to Irritating to skin and mucous membranes. The workshop should be ventilated, the equipment should be sealed, and operators should wear protective equipment.
3. Exist in smoke.
4. When exposed to iron, it appears blue to blue-red-purple.
Storage method
Stored sealed and protected from light.
Aluminum drum packaging, specification 100kg. Store and transport according to regulations on flammable and toxic chemicals.
Synthesis method
1. γ-Butyrolactone and vinyl acetate are produced by Claisen condensation. 2. It is prepared by condensation and ring-closure of ethyl acetoacetate (or methyl acetoacetate) and ethylene oxide. Japan used method 1 to produce it, and the yield was slightly higher than method (2), but it required the use of metallic sodium, which had safety issues. In 1965, it switched to method 2. The process of method 2 is as follows: Add ethylene oxide to a mixture of sodium hydroxide, water and ethanol that has been cooled to below 0°C, add ethyl acetoacetate dropwise, and keep the reaction temperature at about 0°C. After dripping, continue to keep stirring for 5-6 hours, leave it overnight, keep stirring at 0-3°C for 8 hours the next day, and leave it overnight again. On the third day, neutralize with acetic acid to ph=8, and pump with chloroform (or benzene) four times. Extract, combine each extract, recover chloroform (or benzene) and ethanol under normal pressure, and then fractionate under reduced pressure. Above 85℃/5333Pa are low boiling substances (mainly ethyl acetoacetate), 100℃/400-667Pa The above is γ-acetyl butyrolactone, with an average yield of nearly 60% and a crude product content of about 90%.
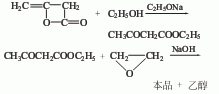
Purpose
1. Organic synthesis. An important intermediate in the synthesis of vitamin B. It is also an intermediate in the synthesis of 3,4-disubstituted pyridine and 5-(β-hydroxyethyl)-4-methylthiazole. Used in the manufacture of vitamin B1 and pain relief drugs

 微信扫一扫打赏
微信扫一扫打赏

