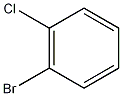
Structural formula
| Business number | 07AB |
|---|---|
| Molecular formula | C6H4BrCl |
| Molecular weight | 191.45 |
| label |
2-Chloro-1-bromobenzene, o-chlorobromobenzene, o-bromochlorobenzene, 2-Bromochlorobenzene, o-Bromochlorobenzene, BrC6H4Cl, organic synthesis intermediates, Multifunctional solvents, aromatic compounds |
Numbering system
CAS number:694-80-4
MDL number:MFCD00000532
EINECS number:211-775-1
RTECS number:None
BRN number:1855303
PubChem number:24891892
Physical property data
1. Properties: colorless liquid.
2. Density (g/mL, 25/4℃): 1.6382
3. Melting point (ºC): -12.3
4. Boiling point (ºC , 101.3kPa): 204
5. Refractive index (20ºC): 1.5804
6. Flash point (ºC): 79
7. Solubility: Easily soluble in benzene, insoluble in water.
Toxicological data
Harmful by inhalation or oral administration, irritating to eyes, skin and mucous membranes, avoid inhalation of vapor.
Ecological data
Generally not hazardous to water, do not discharge material into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 38.83
2. Molar volume (cm3/mol): 117.5
3. Isotonic specific volume (90.2K): 293.6
4. Surface tension (dyne/cm): 38.9
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 15.39
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.2
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 74.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Keep away from oxides.
Storage method
Store in a sealed containerInside, and place in a cool, dry place. Store away from oxidizing agents.
Synthesis method
It is obtained by diazotization of o-chloroaniline and then reacting with copper bromide. Mix o-chloroaniline and 48% hydrobromic acid, add ice to cool down to 0°C, quickly add sodium nitrite aqueous solution while stirring, and add crushed ice to keep the temperature below 10°C. Then the mixture of cuprous chloride and hydrobromic acid is heated to boiling and diazo liquid is added. Control the adding speed of diazo liquid and keep the reaction at boiling. After adding the diazo liquid, the product is evaporated with water vapor, and the organic phase (lower liquid) is separated from the distillate, washed with concentrated sulfuric acid, washed with water, washed with 5% sodium hydroxide solution, and then dried with calcium chloride After distillation, the 199-201℃ (98.4kPa) fraction is collected as the finished product.
Purpose
Used as organic synthesis intermediates and solvents.

 微信扫一扫打赏
微信扫一扫打赏

