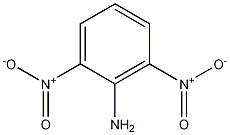
Structural formula
| Business number | 0694 |
|---|---|
| Molecular formula | C6H5N3O4 |
| Molecular weight | 183.12 |
| label |
1-amino-2,6-dinitrobenzene, (O2N)2C6H3NH2 |
Numbering system
CAS number:606-22-4
MDL number:MFCD00007148
EINECS number:210-108-1
RTECS number:BX9200000
BRN number:2214886
PubChem number:24866229
Physical property data
1. Character: yellow needle crystal[1]
2. Melting point (℃): 139~140[2]
3. Solubility: insoluble in water, slightly soluble in ethanol, soluble in ether and hot benzene. [3]
Toxicological data
1. Acute toxicity[4] LD50: 180mg/kg (mouse vein)
2. Irritation No information available
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[5] This substance It is harmful to the environment and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 43.58
2. Molar volume (cm3/mol): 115.3
3. Isotonic specific volume (90.2K ): 344.0
4. Surface tension (dyne/cm): 79.0
5. Polarizability (10-24cm3): 17.27
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 118
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 199
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[6] Stable
2. Incompatible substances [7] Strong oxidants, strong acids, acid anhydrides, acid chlorides
3. Conditions to avoid contact[ 8] Heating
4. Polymerization hazard[9] No polymerization
5. Decomposition products[10] Nitrogen oxides, ammonia
Storage method
Storage Precautions[11] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Packaging seal��Should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
This product is prepared from chlorobenzene as the starting material through the following steps. Keep chlorobenzene, concentrated sulfuric acid and 25% fuming sulfuric acid (volume ratio 6:1) together in a boiling water bath for 2 hours, then cool to room temperature. Add potassium nitrate into the obtained p-chlorobenzenesulfonic acid solution in portions while stirring, and the adding temperature is 40-60°C. After most of the potassium nitrate is dissolved, heat to 110-115°C and keep warm for 20 hours. Pour the nitration solution into crushed ice to freeze, and filter out the yellow precipitate, which is potassium 4-chloro-3,5-dinitrobenzene sulfonate. Dissolve it in boiling water and filter out the insoluble matter. The filtrate was cooled at 5-10°C for 12h. Filter out the crystals, mix with ammonia and reflux for 1 hour. After cooling, filter out the orange potassium 4-amino-3,5-dinitrobenzene sulfonate crystals, and then reflux with sulfuric acid for 6 hours to generate 2,6-dinitroaniline. . The yield is 30-36%.
Purpose
1. Used for the determination of phenol and certain unsaturated hydrocarbons. Dye intermediates.
2. Used in organic synthesis and as analytical reagents. [12]

 微信扫一扫打赏
微信扫一扫打赏

