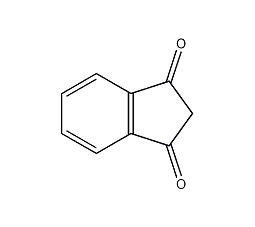
Structural formula
| Business number | 0695 |
|---|---|
| Molecular formula | C9H6O2 |
| Molecular weight | 146.14 |
| label |
1,3(2H)-1H-indanedione, 1H-Indene-1,3-(2H)-dione, Inean-1,3-dione |
Numbering system
CAS number:606-23-5
MDL number:MFCD00003779
EINECS number:210-109-7
RTECS number:NK5070000
BRN number:1210061
PubChem number:24869406
Physical property data
1. Properties: needle-shaped crystals.
2. Density (g/mL, 25/4℃): 1.37
3. Melting point (℃): 131~132℃ (decomposition).
4. Solubility: Slightly soluble in water, soluble in ethanol, ether, benzene and alkali.
Toxicological data
None yet
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 38.68
2. Molar volume (cm3/mol): 111.5
3. Isotonic specific volume (90.2K): 302.8
4. Surface tension (dyne/cm): 54.3
5. Polarizability (10-24cm3): 15.33
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 186
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Nitrate with nitric acid in acetic acid or acetic anhydride to obtain 2-nitro derivatives. Nitro derivatives can be condensed with benzyl alcohol and can also be reduced.
2. Exist in mainstream smoke.
Storage method
Storage:
Seal the container and store it in a sealed main container in a cool, dry place.
Synthesis method
1. Brief description of the production method
It is produced by condensation of diethyl phthalate and ethyl acetate under the action of sodium ethoxide, and then hydrolysis with dilute acid.
2. Preparation method:
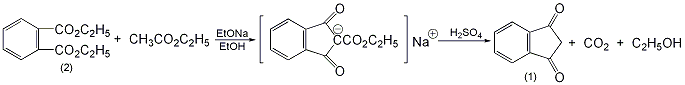
In a reaction bottle equipped with a stirrer, dropping funnel, and reflux condenser (installed with a calcium chloride drying tube), add 125g (0.563mol) of diethyl phthalate (2) and sodium metal ��25g (1.09mol), heated in steam bath. A mixture of 122.5g (1.39mol) of dry ethyl acetate and 2.5mL of absolute ethanol was added dropwise, and the addition was completed in about 1.5h, and then the reaction was continued with stirring for 6h. Cool and add 50 mL of diethyl ether. The precipitated sodium salt was filtered and washed with ethyl acetate. Dissolve the sodium salt in 1.5L hot water, cool to 70°C, and add 100mL sulfuric acid (composed of 3 parts sulfuric acid and 1 part water) with vigorous stirring. Cool to 15°C in ice water bath. Filter, wash with water, and dry at 100°C to obtain 58 g of 1,3-indanedione (1), with a yield of 71%. Recrystallize from dioxane-benzene (add petroleum ether), mp130°C. [1]
Purpose
3. Uses
Used as organic synthesis reagents.

 微信扫一扫打赏
微信扫一扫打赏

