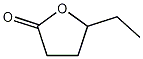
Structural formula
| Business number | 07AF |
|---|---|
| Molecular formula | C6H10O2 |
| Molecular weight | 114.14 |
| label |
γ-ethyl-γ-butyrolactone, coumarolactone, γ-Hydroxycaprolactone, γ-Caprolactone, γ-Ethyl-γ-butyrolactone, Tonkalide, Daily fragrance fixative, spices and essences, food additives, food flavoring agent |
Numbering system
CAS number:695-06-7
MDL number:MFCD00005401
EINECS number:211-778-8
RTECS number:LU4220000
BRN number:107260
PubChem number:24852863
Physical property data
1. Properties: colorless liquid. Mild and powerful herbal vanillin-like aroma, sweet and strong herbal, coumarin, caramel-like taste.
2. Density (g/mL, 25/4℃): 1.027
3. Relative density (20℃, 4℃): 1.027
4 . Melting point (ºC): -18
5. Boiling point (ºC, normal pressure): 219
6. Refractive index at room temperature (n20): 1.4390
7. Refractive index (n20D): 1.439
8. Flash Point (ºC): 98
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol) : Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol /water) logarithmic value of the distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V) : Undetermined
19. Solubility: Slightly soluble in water, soluble in organic solvents such as ethanol and propylene glycol.
Toxicological data
1. Acute toxicity: Rat (orally) LD50: >5mg/kg Rabbit (skin) LD50: >5mg/kg Since the LD50 of table salt is 3,000 mg/kg, the acute toxicity of BPA is the same as that of table salt.
Ecological data
Do not allow undiluted or large amounts of products that are slightly harmful to water.Contact with groundwater, watercourses or sewage systems. Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 29.50
2. Molar volume (cm3/mol): 113.8
3. Isotonic specific volume (90.2K ): 264.1
4. Surface tension (dyne/cm): 29.0
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 11.69
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 98.7
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Keep away from oxides.
2. Exist in flue-cured tobacco leaves, burley tobacco leaves and smoke.
3. Naturally found in apricots, asparagus, wheat bread, pineapples, and peaches.
Storage method
Store in an airtight container in a cool, dry place. Store away from oxidizing agents.
Synthesis method
1. Obtained from the reaction of ethylene oxide and sodium malonate.
2. Tobacco: BU, 56; BU, 14; FC, 9, 18; BU, 18. It is obtained by reducing sorbic acid with tin dichloride and concentrated hydrochloric acid in acetic acid solution at 85ºC.
Purpose
1. It is often used as a modifier of coumarin in flavors, such as in lavender, fragrant and oakmoss as a mossy sweetener. In food flavors, it is widely used in cream, honey, vanilla bean, caramel and fruity compounds and tobacco flavors. Keep away from oxides, moisture, alcohol, amines.
2. Commonly used in baked goods, puddings, and beverages.

 微信扫一扫打赏
微信扫一扫打赏

