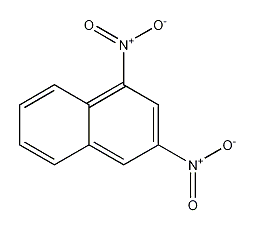
Structural formula
| Business number | 0699 |
|---|---|
| Molecular formula | C10H6N2OS4 |
| Molecular weight | 218.17 |
| label |
dinitronaphthalene, C10H6(NO2)2 |
Numbering system
CAS number:606-37-1
MDL number:MFCD00003915
EINECS number:210-116-5
RTECS number:QJ4550800
BRN number:None
PubChem number:24847821
Physical property data
1. Characteristics: Yellow needle-shaped crystals. 2. Density (g/mL,25℃) : Undetermined 3. Relative vapor density (g/mL,Air=1): Undetermined 4. Melting point (ºC):144- 145 5. Boiling point (ºC,normal pressure): Undetermined 6. Boiling point (ºC, kPa):Undetermined 7. Refractive index (D20):Undetermined 8. Flashpoint (ºC): Undetermined 9. Specific rotation (ºC): Undetermined 10. Autoignition point or ignition temperature (ºC): Undetermined 11. Vapor pressure (mmHg, ºC): Undetermined 12. Saturated vapor pressure (kPa, ºC): Undetermined 13. Heat of combustion (KJ/mol): Undetermined 14. Critical temperature (ºC): Undetermined 15. Critical pressure (KPa): Undetermined 16. Oil and water (octanol/Log value of the partition coefficient for water: undetermined 17. Explosion limit (%,V/V): Undetermined 18. Lower explosion limit (%,V/V): Undetermined 19. Solubility:Soluble in alcohol, insoluble in water. Can be sublimated.
Toxicological data
Ecological data
1. Other harmful effects: This substance may be harmful to the environment and should be harmful to water bodies. Give special attention.
Molecular structure data
1、 Molar refractive index:57.18 2, Molar volume (m3/mol):147.2 3、 Isotonic specific volume (90.2K):422.0 4、 Surface tension (dyne/cm):67.5 5, Polarizability (10-24cm3 ):22.67
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 91.6
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 296
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
can be sublimated. Irritating. Flammable.
Storage method
Should be sealed and stored in a cool place.
Synthesis method
Obtained from naphthalene through mixed acid nitration, acid separation, dehydration and drying. Also available byα-Preparation of nitronaphthalene, α-Nitronaphthalene is dissolved in sulfuric acid, add nitric acid in 80-90℃Nitrification produces dinitronaphthalene.
Purpose
Used as a sensitizer for ammonium nitrate explosives (the actual product is a mixture of isomers), carbide additives, and intermediates for sulfur dyes and organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

