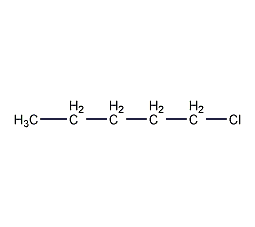
Structural formula
| Business number | 05M3 |
|---|---|
| Molecular formula | C5H11Cl |
| Molecular weight | 106.59 |
| label |
Pentyl chloride, Pentyl chloride, 1-Chloropentane, Chlorinated n-pentane, n-Amyl chloride, n-Pentyl chloride, refining agent, For the synthesis of pentanol, Halogenated hydrocarbon solvents |
Numbering system
CAS number:543-59-9
MDL number:MFCD00001015
EINECS number:208-846-4
RTECS number:None
BRN number:1696936
PubChem number:24854299
Physical property data
1. Properties: colorless or light yellow liquid with fragrance.
2. Boiling point (ºC, 101.3kPa): 108.4
3. Melting point (ºC): -99.0
4. Relative density (g/mL, 20/4ºC): 0.8820
5. Refractive index (n20ºC): 1.4126
6. Viscosity (mPa·s, 20ºC): 0.580
7. Flash point (ºC, open): 12.2
8. Fire point (ºC): 259
9. Heat of vaporization (KJ/mol, b.p.): 32.74
10. Heat of combustion (KJ/mol): 3064.3
11. Heat of formation (KJ/mol, 298.16K, liquid): 212.3
12. Heat of formation (KJ/mol , 298.16K, liquid): 173.8
13. Specific heat capacity (KJ/(kg·K), 20ºC, constant pressure): 1.84
14. Critical temperature (ºC): 289
15. Critical pressure (MPa): 3.42
16. Vapor pressure (kPa, 25ºC): 4.14
17. Lower explosion limit (%, V/ V): 1.6
18. Explosion upper limit (%, V/V): 8.6
19. Volume expansion coefficient (K-1, 20ºC) : 0.001208
20. Relative density (25℃, 4℃): 0.8771
21. Refractive index at room temperature (n25): 1.4104
22. Solubility: Soluble in alcohol, ether, ethyl acetate, acetone, oil, oleic acid, aromatic hydrocarbons, aliphatic hydrocarbons, etc. It is also soluble in stearic acid and paraffin when heated. Can dissolve wax, tar and resin, etc. Almost insoluble in water, the solubility in water at 25℃ is 0.020%.
23. Solubility parameter (J·cm-3)0.5: 16.952
24. van der Waals area ( cm2·mol-1): 9.320×109
25. van der Waals volume (cm3·mol-1): 66.210
26. Gas phase standard claims heat (���)( kJ·mol-1): -175.18
27. The liquid phase standard claims heat (enthalpy) ( kJ·mol-1 ): -213.44
28. Liquid phase standard hot melt (J·mol-1·K-1): 187.3
Toxicological data
None yet
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 30.06
2. Molar volume (cm3/mol): 122.4
3. Isotonic specific volume (90.2K ): 269.5
4. Surface tension (dyne/cm): 23.4
5. Polarizability (10-24cm3): 11.91
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 19.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
A flammable liquid that produces highly toxic phosgene when decomposed by heat. The boiling point of the azeotropic mixture with water is 82°C, and the boiling point of the azeotropic mixture with ethanol is 72.5°C. Under the catalysis of sodium oleate, it is hydrolyzed in sodium hydroxide aqueous solution to generate pentanol. Heated with concentrated hydrochloric acid and zinc chloride to 126~134°C, partial isomerization generates a mixture of 2-chloropentane and 3-chloropentane.
Storage method
Stored in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from acids and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Obtained from chlorination of pentane. First dehydrate the pentane with hydrogen chloride, evaporate it in the evaporator, mix it with chlorine gas at a pressure of 0.55MPa at a high flow rate of 2000dm3 in a Venturi mixer made of pig iron, control the temperature to 50°C, and then send the mixed gas into in a tubular evaporator reactor. The molar ratio of pentane to chlorine is 15:1. The reactor temperature was 120°C at the steam inlet and gradually increased to 300°C. The product is immediately sent to the cooler for cooling to inhibit the formation of dichloropentane. The product consists of unreacted pentane, hydrogen chloride, chloropentane and a small amount of dichloropentane. After cooling and fractional distillation to remove hydrogen chloride and pentane, the finished product of 1-chloropentane is obtained. Another preparation method is to react pentanol with hydrogen chloride. First use hydrochloric acid to dissolve zinc chloride, then add pentanol, reflux at 120°C for 3 hours and then distill. The crude product obtained is washed and fractionated, and the 106-109°C fraction is collected as the finished product. .
Purpose
Organic synthesis intermediates, solvents. Mainly used in the manufacture of amyl alcohol. Used as a refining agent in the fiber industry.

 微信扫一扫打赏
微信扫一扫打赏

