Background and overview[1]
Boron trifluoride complex is a highly active catalyst that can be used in a variety of organic synthesis reactions, especially in the synthesis process of cephalosporin antibacterial drugs. Due to the introduction of boron trifluoride complex , shortening the reaction time of the original product, and greatly improving the yield of the original product. The catalytic effect of boron trifluoride acetonitrile complex is better than that of boron trifluoride etherate complex, so boron trifluoride acetonitrile complex has broad market prospects.
Preparation[1]
Currently, the fluorite boric acid method is mostly used in China to produce boron trifluoride gas. This method uses fluorite as the reaction raw material during the production process. After the reaction, a large amount of calcium sulfate solid waste will be produced, causing very serious pollution to the environment. , and at the same time, using this method for production requires high labor intensity and affects the health of production personnel; in addition, the fluorite boric acid method is an intermittent reaction and has low output. CN201310296394.5 provides a method for preparing boron trifluoride acetonitrile with high yield and less pollution.
The process is as follows:
(1) Mixed acid: Use fuming sulfuric acid and boric acid as raw materials to react to generate boron trioxide;
(2) Gas generation reaction: Preheat the fluorination reaction tower. When the temperature reaches 250°C, start using a metering pump to transport the mixed acid solution into the tower. At the same time, open the hydrogen fluoride cylinder and introduce hydrogen fluoride for reaction to produce boron trifluoride gas. , the equation of fluorination reaction is:
![]()
(3) Purification: Filter and purify the boron trifluoride gas obtained through sulfuric acid in the purification container;
(4) Complexation reaction: Preheat acetonitrile for use. The preheating temperature is 60°C. When the boron trifluoride gas is generated and reaches the complexing tank, acetonitrile is added for complexation. The complexed trifluoride The boron acetonitrile complex flows directly into the finished product receiving tank to prepare the finished product.
Figure 1 is a process flow chart for preparing boron trifluoride acetonitrile.
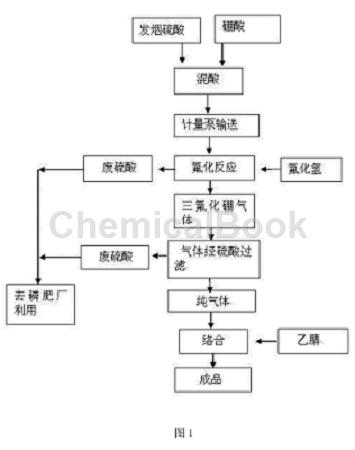
Beneficial effects of the present invention: use fuming sulfuric acid and boric acid to react to produce diboron trioxide, and then react diboron trioxide with hydrogen fluoride to produce boron trifluoride gas. It is highly safe and has no waste residue. The sulfuric acid is supplied to the phosphate fertilizer plant. Secondary utilization simultaneously reduces the labor intensity of employees and is beneficial to the physical and mental health of employees. In addition, the products produced by the invention are stable and have high yields.
Apply [2-4]
1. Used in the preparation of cefotiam hydrochloride
Cefotiam hydrochloride is a second-generation injectable antibiotic. Its effect on Gram-positive bacteria is similar to that of cefazolin. It has good effects on Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Haemophilus. It also has antibacterial effects on Citrobacter, Enterobacter, and Indole-positive Proteobacteria. CN201410650034.5 provides a method for preparing cefotiam hydrochloride, which is achieved through the following technical solution: using 7-ACA and DMMT as raw materials, boron trifluoride acetonitrile complex as the catalyst, and acetonitrile as the reaction solvent. The 7-ACMT reaction solution is prepared by the condensation reaction; this reaction solution does not need to be separated and purified 7-ACMT, it can be directly added with water, adjusted to alkali, and then “conducted” with ATC·HClOne-pot “acylation reaction”; after the acylation reaction solution is acidified and extracted with organic solvent to separate organic impurities, a hydrophilic solvent is added to precipitate high-purity cefotiam hydrochloride.
2. Used in the preparation of cefoperazone acid
Cefoperazone is a third-generation cephalosporin antibiotic with a broad antibacterial spectrum and is the first-line drug against Pseudomonas aeruginosa. CN201310625115.5 provides a method for preparing cefoperazone acid. The reaction does not require the preparation of acid chloride from HO-EPCP. Homemade benzotriazolyl diethyl phosphate (BDP) is directly added to directly react HO-EPCP with the protected 7 -TMCA hydrochloride performs acylation reaction with simple reaction steps, low cost and high purity.
The preparation method of cefoperazone acid according to the present invention includes the following steps:
(1) Under the catalysis of boron trifluoride acetonitrile, use 7-ACA and 1-methyl-5-mercaptotetrazole as raw materials to prepare 7-TMCA hydrochloride, and then use trimethyl chloride Silane protects the carboxyl and amino groups of 7-TMCA hydrochloride;
(2) Using the group-protected 7-TMCA hydrochloride, HO-EPCP and benzotriazolyl diethyl phosphate prepared in step (1) as raw materials, under the catalysis of triethylamine, N-acylation reaction is carried out in DMF solution to obtain cefoperazone acid.
3. New synthesis process for 7-ACT
7-ACT, also known as 7-aminoceftriazoline, is an intermediate in the synthesis of ceftriaxone sodium. The quality of 7-ACT products will directly affect key quality index parameters such as color grade, content, single impurities, and total impurities of the final product. Therefore, 7-ACT is the key to affecting the quality of this type of cephalosporin antibiotic finished product. CN201711182027.7 provides a new synthesis process of 7-ACT, including the following steps:
(1) After mixing acetonitrile and boron trifluoride acetonitrile complex, add 7-ACA and triazine ring, stir the reaction at 10℃~20℃ until the 7-ACA residue is ≤0.5% as detected by liquid chromatography. , the reaction is considered complete;
(2) Reduce the reaction solution to 0~10℃, add 0~10℃ purified water, and hydrolyze;
(3) After adding ethyl acetate for extraction, add ethyl acetate to the water layer for secondary extraction;
(4) Take the water layer and add alkali solution dropwise at 0 to 10°C to adjust the pH to 3.4 to 3.6. During the dripping process, control the temperature not to exceed 10°C. After the dripping is completed, stir and grow the crystals at 0 to 10°C for 2 hours. ;
(5) Filter, wash twice and dry to obtain 7-ACT.
Main reference materials
[1] CN201310296394.5 Preparation method of boron trifluoride acetonitrile
[2] CN201410650034.5 Preparation method of cefotiam hydrochloride
[3] CN201310625115.5 Preparation method of cefoperazone acid
[4] CN201711182027.7 New synthesis process of 7-ACT

 微信扫一扫打赏
微信扫一扫打赏

