Background and overview[1]
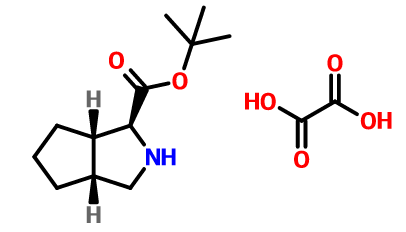
(1S,3AR,6AS)-Otahydrocyclopenta[C]pyrrole-1-carboxylic acid tert-butyl ester oxalate is an S-configured nitrogen heterocycle and an intermediate of telaprevir. S-configuration nitrogen heterocycles are widely concerned as common compounds in stereochemistry and important intermediates for various drugs. (1S,3AR,6AS)-Otahydrocyclopenta[C]pyrrole-1-carboxylic acid tert-butyl ester oxalate can be made from cis-7-azabicyclo[3.3.0]octane hydrochloride. It is prepared through a five-step reaction.
Preparation[1]
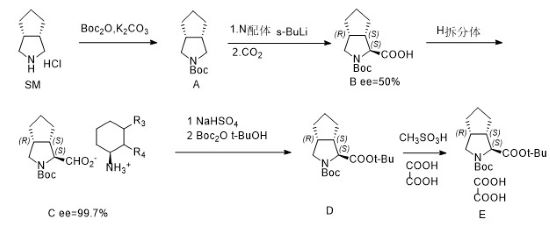
Step 1:
Dissolve 131kg of K2CO3 into 125kg of water, add 25kg of compound SM and 125kg of n-heptane, stir for 1 hour, lower the temperature to 0-15°C, control the temperature to 0-15°C, and slowly drop 326kg of B°C2O into the reaction system , after the addition is completed, keep stirring at 0-15°C for 3 hours. After the reaction is completed, the obtained organic layer is concentrated to obtain compound A.
Step 2/Step 3:
Add 70kg of compound A, 98kg of N ligand and 400kg of methyl tert-butyl ether into the reaction kettle, cool the system to -80~-70°C, and slowly add 312kg of 1.2mol /L sec-butyllithium solution, after adding it, keep it warm and stir for 3 hours. Pour in 35kg CO2, keep warm and stir for 1 hour after adding. After the reaction is completed, heat the system to 0-10°C to obtain compound B (yield: 90%, S:R=75:25). Add 450kg of water and separate the liquids. Use 3NHCl to adjust the pH of the water phase to 3.0-3.5. After the adjustment is completed, extract with methyl tert-butyl ether and concentrate the methyl tert-butyl ether. After concentration, add 90kg of toluene and heat the system. to 30-40°C, add 50kg of chiral compound H, reflux the system for 3 hours, slowly lower the temperature to 10-20°C and keep stirring for 2-3 hours. Compound C was obtained by centrifugation (two-step yield: 50%, ee=100%).
Step 4/Step 5:
Add 100kg compound C to 200kg MTBE, add 400kg 0.5N hydrochloric acid solution, stir at 0-15°C for 0.5 hours, separate the liquids, wash the organic phase with water, add 84kg tert-butanol and 5.7kg DMAP, and add dropwise (B°C) 2O, complete the addition, stir for 5 hours, the reaction is completed, the reaction solution is washed with sodium bisulfate solution and sodium chloride solution, add 71.6kg tert-butanol and 57kg methanesulfonic acid, stir for 11 hours, the reaction is completed, add 400kg of 30% Potassium carbonate solution, stir for 0.5 hours, separate the liquids, concentrate the organic phase until no solvent is distilled, add 250kg ethyl acetate and 63.2kg methanol, control the system temperature to 0-10°C, add 25kg oxalic acid and 240kg methyl tert-butyl dropwise Ether solution. After the dropwise addition is completed, keep warm and stir for 7-8 hours. Centrifuge and rinse the filter cake with an appropriate amount of ethyl acetate. The filter cake is dried under reduced pressure between 35-45°C for 16-17 hours or there is no change in weight. That is, compound E ((1S,3AR,6AS)-octahydrocyclopenta[C]pyrrole-1-carboxylic acid tert-butyl ester oxalate) was obtained.
References
[1] [Chinese invention] Preparation method of CN201510519658.8 (1S,3aR,6aS)-octahydrocyclopenta[C]pyrrole-1-carboxylic acid tert-butyl ester oxalate

 微信扫一扫打赏
微信扫一扫打赏

