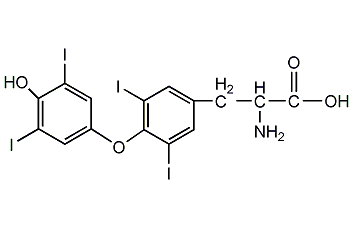
Structural formula
| Physical competition number | 014X |
|---|---|
| Molecular formula | C15H11I4NO4 |
| Molecular weight | 776.87 |
| label |
2-Thyroxine, O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo-L-tyrosine, D-alpha-tocopherol, levothyroxine, 3,5,3′,5′-Tetraiodothyronine, 3,3′,5,5′-Tetraiodo-L-thyronine |
Numbering system
CAS number:51-48-9
MDL number:MFCD00002595
EINECS number:200-101-1
RTECS number:YP2833500
BRN number:2228515
PubChem ID:None
Physical property data
1. Properties: white crystal.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Dissolved in alkali solution, Insoluble in water, ethanol and ether
4. Melting point (ºC): 235
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation (º): -4.45 (546nm) (EtOH/NaOH)
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure ( kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC):
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility:. Undetermined
Toxicological data
1. Acute toxicity: Oral TDLo for men: 63ug/kg; Oral TDLo for women: 400ug/kg/2D-I2. Other multi-dose toxicity: Rat subcutaneous TDLo: 2mg/kg/10D-I3. Reproductive toxicity: Female Rat oral TDLo: 26250ug/kg, conception takes 1-21 days; female rat oral TDLo: 1400ug/kg, conception takes 16-22 days; Female rat oral TDLo: 410ug/kg, conception takes 1-20 days; female Rat subcutaneous TDLo: 410ug/kg, conception occurs after 7-15 days;
Ecological data
None
Molecular structure data
1. Molar refractive index: 125.44
2. Molar volume (cm3/mol): 294.7
3. Isotonic specific volume (90.2K ���: 880.4
4. Surface tension (dyne/cm): 79.6
5. Polarizability (10-24cm3): 49.73
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.4
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: 2
6. Topological molecule polar surface area 92.8
7. Number of heavy atoms: 24
8. Surface charge: 0
9. Complexity: 420
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure
Avoid light, open flame, high temperature,
Storage method
Should be sealed with argon and stored in a cool, dry place away from light
Synthesis method
Can be extracted from animal thyroid glands. It can be prepared from 3,5-diiodo-L-tyrosine.
The formation of thyroxine goes through six processes: synthesis, storage, iodination, reabsorption, decomposition and release: 1. Follicular epithelial cells absorb amino acids from the blood and synthesize thyroid spheres in the rough endoplasmic reticulum. The precursor of the protein is then added with sugar in the Golgi complex and concentrated to form secretory granules, which are then discharged into the follicular cavity for storage by exocytosis. 2. Follicular epithelial cells can absorb I- from the blood, and I- is activated through the action of peroxidase. 3. The activated I- enters the follicular cavity and combines with thyroglobulin to form iodinated thyroglobulin. 4. Under the action of thyroid-stimulating hormone secreted by the adenohypophysis, follicular epithelial cells endocytose iodinated thyroglobulin in the follicular cavity and become glial vesicles. 5. Glial vesicles fuse with lysosomes, and iodinated thyroglobulin is decomposed by hydrolase to form a large amount of tetraiodothyronine (T4) and a small amount of triiodothyronine (T3), which is thyroxine. 6. T3 and T4 are released into the blood at the base of cells.
Purpose
Biochemical research; thyroxine drugs
extended-reading:https://www.bdmaee.net/polycat-12-catalyst-cas10144-28-9-evonik-germany/extended-reading:https://www.newtopchem.com/archives/39611extended-reading:https://www.bdmaee.net/wp-content/uploads/2016/06/Jeffcat-ZF-22-MSDS.pdfextended-reading:https://www.bdmaee.net/potassium-neodecanoate-2/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/N-Formylmorpholine-CAS4394-85-8-4-formylmorpholine.pdfextended-reading:https://www.newtopchem.com/archives/44720extended-reading:https://www.newtopchem.com/archives/44300extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/129-1.jpgextended-reading:https://www.newtopchem.com/archives/category/products/page/50extended-reading:https://www.cyclohexylamine.net/foam-amine-catalyst-strong-blowing-catalyst/

 微信扫一扫打赏
微信扫一扫打赏

