background and overview[1]
3-acetonitrilecyclobutylamine hydrochloride can be used as a pharmaceutical synthesis intermediate.
preparation[1-2]
method 1: 3-acetonitrilecyclobutylamine hydrochloride is prepared as follows:
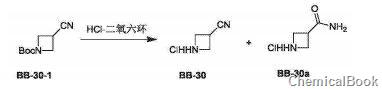
compound bb-30-1 (0.200g, 1.10mmol) was dissolved in dichloromethane (2ml), and then hydrogen chloride dioxane solution (1ml, 4m) was added. the reaction solution was stirred at 20°c for 2 hours. the reaction solution was concentrated to obtain target compounds bb-30 and bb-30a (white solid, 0.130 g, yield: 99%), which were directly used in the next step of synthesis without isolation. ms(esi) m/z: 83[m+h]+. ms(esi)m/z: 101[m+h]+.
method 2: 3-acetonitrilecyclobutylamine hydrochloride is prepared as follows:
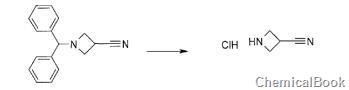
to a 50 ml flask, add 1-diphenylmethylazetidine-3-carbonitrile (500 mg, 2.01 mmol) and 1,2-dichloroethane (8.9 ml). 1-chloroethyl chloroformate (285 μl, 2.61 mmol) was added and the reaction mixture was stirred at 70°c for 1.5 hours. after cooling to room temperature, methanol (8.9 ml) was added and the reaction mixture was stirred at 70°c for 1.5 hours. the reaction mixture was concentrated to dryness. the crude mixture was triturated in pentane to give a black solid (250 mg, quantitative).
apply[1]
3-acetonitrilecyclobutylamine hydrochloride can be used to prepare tert-butyl 3-cyanoazetidine-1-carboxylate:

to a solution of azetidine-3-carbonitrile hydrochloride (250mg, 2.01mmo) and triethylamine (1.12ml, 8.04mmol) in dichloromethane (10.2ml) at 5°c. di-tert-butyl dicarbonate (482 mg, 2.21 mmol) was added in one portion. after stirring at room temperature for 16 hours, the reaction mixture was washed with 0.5 n hcl and brine and dried over sodium sulfate. the solvent was evaporated under reduced pressure and the crude mixture was purified by column chromatography (eluent: pentane/etoac 95/5 to 4/1) to give the title compound (190 mg, 52%) as a colorless oil. 1hnmr (cdcl3, 300mhz): δ (ppm): 4.29-4.05 (m, 4h), 3.48-3.29 (m, 1h), 1.44 (s, 9h).
main reference materials
[1] cn102656158 – new heterocyclopropylamide compounds and their use as drugs
[2] cn201610070866.9 benzofuran analogues as ns4b inhibitors

 微信扫一扫打赏
微信扫一扫打赏

