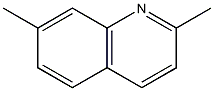
Structural formula
| Business number | 0266 |
|---|---|
| Molecular formula | C11H11N |
| Molecular weight | 157.21 |
| label |
2,7-Methylquinaldine, m-Toluquinaldine |
Numbering system
CAS number:93-37-8
MDL number:MFCD00006763
EINECS number:202-242-4
RTECS number:None
BRN number:None
PubChem number:24848739
Physical property data
1. Properties: needle-like crystals.
2. Density (g/mL, 25/4℃): 1.0611.
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 61
5. Boiling point ( ºC, normal pressure): 264~265
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: 1.6075.
8. Flash point (ºC): Not determined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
p>
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper limit of explosion (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in ethanol, ether and benzene.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 51.83
2. Molar volume (cm3/mol): 149.3
3. Isotonic specific volume (90.2K ): 380.5
4. Surface tension (dyne/cm): 42.1
5. Polarizability (10-24cm3): 20.54
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 12.9
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 155
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and Stability
None
Storage method
This product should be sealed and stored away from light.
Synthesis method
Heat the aniline with iodine to 170-175°C, add acetone dropwise, and stir vigorously while collecting the distillate. Aniline and the middle fraction 2,2,4 trimethyl-1,2-dihydroquinoline are separated from the reaction mixture and distillate by distillation under reduced pressure. The latter is heated and reacted with anhydrous aniline, metallic sodium and copper powder, and fractionated under reduced pressure from the reactants to obtain 2,4-dimethylquinoline with a yield of 80-90%.
Purpose
Used in organic synthesis. Dye intermediates.
extended-reading:https://www.bdmaee.net/niax-ef-712-low-emission-tertiary-amine-catalyst-momentive/extended-reading:https://www.newtopchem.com/archives/44310extended-reading:https://www.newtopchem.com/archives/1769extended-reading:https://www.bdmaee.net/teda-catalyst-triethylene-diamine-tosoh/extended-reading:https://www.cyclohexylamine.net/cas-67874-71-9-bismuth-octoate-bismuth-2-ethylhexanoate/extended-reading:https://www.bdmaee.net/lupragen-n201-catalyst-basf/extended-reading:https://www.bdmaee.net/pc-cat-np-90-catalyst/extended-reading:https://www.bdmaee.net/niax-k-zero-3000-trimer-catalyst-momentive/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dibutyltin-diacetate-CAS1067-33-0-dibutyl-tin-diacetate.pdfextended-reading:https://www.newtopchem.com/archives/1859

 微信扫一扫打赏
微信扫一扫打赏

