[Background and Overview][1][2][3]
4,5-bisdiphenylphosphine-9,9-dimethylxanthene (Xantphos) is an organophosphorus compound derived from xanthene heterocycle. 4,5-bisdiphenylphosphine-9,9-dimethylxanthene is a commonly used bidentate ligand. It is worth noting that this phosphine ligand has a particularly wide bite angle. This ligand is often used in the hydroformylation of alkenes. 4,5-bisdiphenylphosphine-9,9-dimethylxanthene can form both cis and trans adducts with palladium chloride, which proves its wide bite angle . A related bidentate ligand with a larger bite angle is SPANphos. The 4,5-bisdiphenylphosphine-9,9-dimethylxanthene ligand can be prepared by the following method: 9,9-dimethylxanthene is first subjected to double direct lithiation, and then combined with chlorobis Prepared by the reaction of phenylphosphine. 4,5-bisdiphenylphosphine-9,9-dimethylxanthene CAS number is 161265-03-8, molecular formula C39H32OP2, molecular weight 578.61800, white to light yellow crystal, melting point 224-228 °C (lit. ), boiling point 665.7ºC at 760 mmHg, flash point 449.9ºC, vapor pressure 7.61E-17mmHg at 25°C. If inhaled, move the patient to fresh air; in case of skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if eye contact occurs, separate the eyelids and rinse with fluid Rinse with water or saline and seek medical attention immediately. If ingested, rinse mouth immediately. Do not induce vomiting and seek medical attention immediately.
【Synthesis】[1]
Under anhydrous and oxygen-free conditions, 9,9-dimethylxanthene and sec-butyllithium react in a dehydrated diethyl ether solvent to remove the two hydrocarbons on the benzene ring to generate dilithium 9 , 9-dimethylxanthene. In diethyl ether as the solvent, dilithium 9,9-dimethyloxanthene further reacts with diphenylphosphine chloride to generate 4,5-bisdiphenylphosphine-9,9-dimethyloxanthene. anthracene. This compound can be stored in the air for a long time, is easily soluble in methylene chloride, and is insoluble in petroleum ether and other solvents. The reaction formula is as follows:
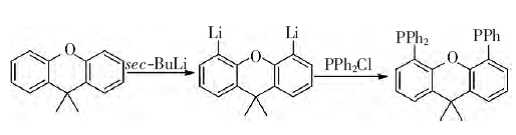
Experimental steps: In a 250 mL three-necked flask, under nitrogen protection, add 2.00 g of 9,9-dimethylxanthene and 3.41 g of TMEDA (tetramethylethylenediamine), and then add 70 mL Dissolve it with diethyl ether. Then add 22 mL of sec-butyllithium with a concentration of 1.3 mol/L dropwise into the reaction bottle through a constant pressure dropping funnel, and stir at room temperature for 16 h. Then add 5.2 mL of sec-butyllithium with a concentration of 5.58 mol/L. The diethyl ether solution of diphenylphosphine chloride was dropped into the reaction bottle drop by drop, and stirred at room temperature for 16 h. Add 20 mL of distilled water for hydrolysis, stir for 10 min, and separate the liquids. The aqueous layer was extracted three times with ethyl acetate, and the organic matter was combined. layer, the organic layer was washed once with water and once with saturated sodium chloride solution, dried over anhydrous sodium sulfate, and the solvent was evaporated to remove the solvent to obtain a yellow oil. Then, 4.1 g of white 4,5-bis was obtained by recrystallization with ethanol. Diphenylphosphine-9,9-dimethylxanthene solid, yield 74%.
[Application][2][3]
4,5-Bisdiphenylphosphine-9,9-dimethylxanthene is mainly used as a ligand in coupling reactions such as Buchwald and Suzuki.
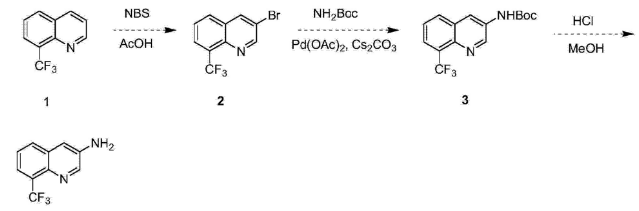
1. Used in the synthesis of 3-amino-8-trifluoromethylquinoline: 3-amino-8-trifluoromethylquinoline and its intermediates are important intermediates in organic synthesis and medicinal chemistry. Starting from these compounds, a series of candidate drug molecules with good pharmaceutical activity can be synthesized. The latest research shows that these candidate molecules, in addition to having anti-angiogenesis, anti-coagulation, anti-tumor and anti-tumor metastasis, and anti-diabetic retinal detachment activities, can also be used to treat diseases such as arthritis and irritable bowel syndrome. The synthesis method is as follows: 8-trifluoromethylquinoline reacts with N-bromosuccinimide in acetic acid to generate 3-bromo-8-trifluoromethylquinoline, and then reacts with tert-butyl carbamate in acetic acid. In the presence of palladium, 4,5-bisdiphenylphosphine-9,9-dimethylxanthene and cesium carbonate, the reaction generates 3-tertiary oxycarbonylamino-8-trifluoromethylquinoline. Finally, the compound is Deamination protection in a mixed solvent of hydrochloric acid and methanol gave 3-amino-8-trifluoromethylquinoline. The reaction equation is as follows:
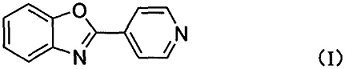
2. Preparation of a new yellowish green phosphorescent mixed cuprous complex luminescent material, the yellowish green phosphorescent mixed cuprous complex Cu (Xantphos)(4-PBO)(CH3CN)(PF 6) Luminescent materials, in which the benzoxazole group introduced is conducive to molecular excited state luminescence, and the existence of charge transition from metal Cu to ligand effectively promotes intersystem crossing. On the other hand, large The bisphosphine chelating ligand Xantphos causes a large steric hindrance around Cu(I), and the overall coordination structure is more rigid, which can suppress the non-radiative attenuation of the excited state of the molecule, so the molecular material has good phosphorescence. Launch performance. This complex material not only has the advantages of being cheap and easy to purify, but also has good solubility, which provides technical support for the further application of luminescent materials. The specific method is obtained by the coordination reaction between Cu(CH3CN)4PF6 and the ligand. Its molecular structure is Cu(Xantphos )(4-PBO) (CH3CN)(PF6), where Xantphos and 4-PBO are the electrically neutral ligands 4,5-bisdiphenylphosphine-9,9- respectively. Dimethylxanthene and 4-(2-benzoxazole)pyridine. The ligand 4-(2-benzoxazole)pyridine is a combination of benzoxazole and pyridinebody, its molecular structure is as follows:
[Main reference materials]
[1] Wang Bin, Han Xianzhu, Liu Kun, Yan Yu, Xia Zhongyue, Liu Wangsheng, Shen Yeye, Zhao Pu, Xu Jincheng. 1,10-phenanthroline and 4,5-bisdiphenylphosphine Synthesis and characterization of -9,9-dimethylxanthene mixed coordination copper (I) Journal of Tianjin Normal University (Natural Science Edition), Volume 34, Issue 3, 2014.
[2] Wang Ling; Xu Weiliang; Xu Weizheng. Synthesis method of amino-8-trifluoromethylquinoline. CN201410363728.0, application date 2014-07-29
[3] Chai Wenxiang; Lin Qizhong; Jia Guohua; Guo Bing; Hong Mingwei; Zhang Zhu; Complex luminescent materials. CN201410341572.6, application date 2014-07-14

 微信扫一扫打赏
微信扫一扫打赏

