[Background and Overview][1][2]
As an important fine chemical intermediate, trifluoromethylbenzene products are currently in the ascendant in terms of their synthesis process and downstream product application research and development, becoming a hot product in the field of fine chemical intermediates. The selection of fluorine atoms and fluorine-containing substituents The introduction of sex will change the physical and chemical properties and physiological activity of the compound, and it plays an important role in drug research. At present, organic fluorides are receiving more and more attention as pharmaceutical intermediates, especially in the development of new anti-cancer drugs.
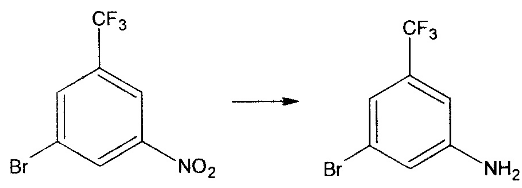
3-Amino-5-bromotrifluorotoluene CAS number 54962-75-3, chemical formula C7H5BrF3N , molecular weight 240.02100, Chinese alias 3-bromo-5-(trifluoromethyl)aniline; 3-trifluoromethyl-5-bromoaniline; 3-bromo-5-trifluoromethylaniline; 3-bromo-5- Aminobenzotrifluoride, light yellow transparent liquid, boiling point 220-223 °C (lit.)), flash point >230 °F, density 1.697 g/mL at 25 °C (lit.), refractive index n20/D 1.528( lit.). 3-Amino-5-bromotrifluorotoluene provides a good intermediate for the development of new anti-cancer drugs. If 3-amino-5-bromotrifluorotoluene is inhaled, move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel unwell; if In case of eye contact, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
【Synthesis】[2]
Using 4-bromo-2-trifluorotoluidine as raw material, 3-amino-5-bromotrifluorotoluene is prepared by acetylation, nitration, deacetylation, deamination and reduction in sequence.
For the acetylation described in 1), put 15 grams of acetic acid into a dry bottle, add 33.6 grams of acetic anhydride under stirring, and add dropwise 4-bromo-2-trifluorotoluidine or 3 – 50 grams of bromo-6-aminotrifluorotoluene, after dripping, raise the temperature to 60 degrees, and keep it warm until the raw material is gone (liquid phase). If the reaction is slow, heat it to reflux, keep it warm until the raw material disappears, pour it into ice water, separate the solid, and pump it out Strain, wash and dry. The yield is 98% and the content is 99.6%.
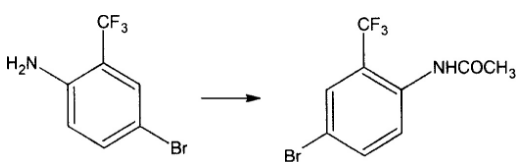
2) For the nitrification described in the dry reaction pot, put 25 grams of sulfuric acid into the reaction pot. Add 10 grams of the above materials into the reaction pot under stirring and stir for 30 minutes at a temperature below 20 degrees. Cool to 10 degrees and add 2 grams of nitric acid dropwise. , until the ingredients disappear, then pour into the water. The solid is precipitated, filtered and washed with water until neutral. After drying, 11.3 grams of dry product was obtained with a yield of 97.4% and a content of 99.4%. Reaction:
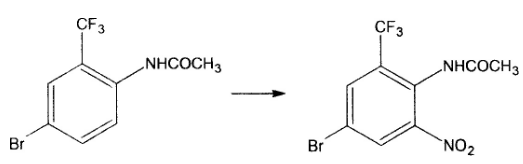
3) For the deacetylation described above, put 10 grams of water into the reaction bottle, add 10 grams of 30% hydrochloric acid and 10 grams of nitrated material while stirring, slowly heat up and reflux, take samples until the amide is completely hydrolyzed, and use ammonia water as the material. Adjust to alkaline, suction filter below 30 degrees, wash and dry. 8.58 grams of dry product was obtained, with a yield of 98.5% and a content of 99.35%. Reaction:
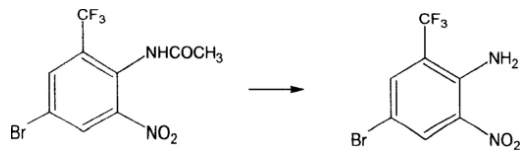
For the deamination described in 4), add 200 ml of sulfuric acid, add 21 grams of sodium nitrite (added to the sulfuric acid in batches, the temperature is 10 degrees), add 70.68 grams of 0.248 mol of the above material dropwise, and the time is 1 hour , put it in an ice bath overnight. Within 2 hours, slowly add the above liquid to 100 ml of 85% phosphoric acid, the temperature should not exceed 20 degrees, and stir for 15 minutes after adding. Then, under rapid stirring, 150 ml of 45% sodium hypophosphite solution was added dropwise to the solution, and the temperature did not exceed 20 degrees. After the addition is completed, add 20 grams of copper oxide in batches, the temperature does not exceed 30 degrees, stir for 15 minutes after the addition, add equal volume of condensed water, filter, separate the material, and wash with alkali to obtain 36 grams of product, yield 53.76%, content 98.53%. Reaction:
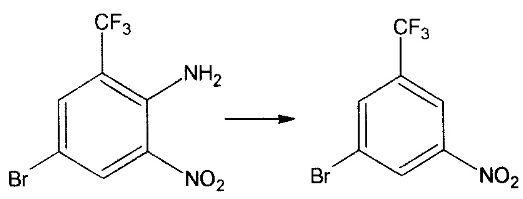
5) For the reduction described in 100 ml reaction pot, add 18 grams of iron powder, 52 ml of water, and 1 ml of glacial acetic acid, and heat and reflux for 30 minutes. Lower the temperature to 90-95 degrees, start adding 20 grams of raw materials dropwise, and after the dripping, raise the temperature and reflux for 2 hours to steam. Get 15.2 grams. Yield: 85.5% Content: 98.5%. Reaction:
[Application]
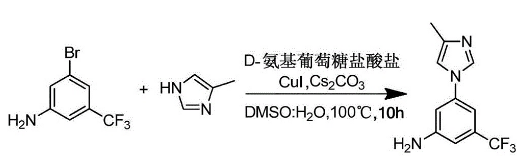
3-Amino-5-bromotrifluorotoluene can be used as an intermediate in the synthesis of nilotinib intermediate 3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl) )aniline. Nilotinib is a new type of ATP competitive inhibitor based on aminopyrimidines and has high affinity. Nilotinib can combine with ABL tyrosine kinase to promote the folding of the P-ring, thereby covering the binding site of ATP, hindering the binding of the substrate, and unable to successfully complete the phosphorylation of ATP, thereby inhibiting the activity of the enzyme. Generates an inactive conformation of the BCR/ABL protein. Nilotinib hydrochloride hydrate can be used to treat chronic myelogenous leukemia that has failed to respond to imatinib mesylate, and selectively inhibit Philadelphia chromosome-positive chronic myeloid leukemia caused by tyrosinase mutations. 3-(4-Methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline is a key intermediate in the synthesis of nilotinib. The preparation method of nilotinib intermediate 3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline includes the following steps:
1) Add 3-amino-5-bromotrifluorotoluene, 4-methylimidazole, and D-aminotrifluorotoluene in sequence in the reaction vessel.�� Glucose hydrochloride, CuI, Cs2CO3, DMSO and water, react at 90~110℃ for 8~12 hours. The molar ratio of the 3-bromo-5-trifluoromethylaniline, 4-methylimidazole, Cs2CO3, D-glucosamine hydrochloride and CuI is 1:1:1.8 ~2.2: 0.05 ~ 0.2: 0.05 ~ 0.2. The dosage of DMSO is 0.5~0.8L/mol 3-bromo-5-trifluoromethylaniline. The amount of water used is 0.5 to 0.8L/mol 3-bromo-5-trifluoromethylaniline.
2) Add ethyl acetate to the reaction solution obtained in step (1), centrifuge and remove the supernatant, concentrate and dry to obtain 3-(4-methyl-1H-imidazol-1-yl)-5-( Trifluoromethyl)aniline. The feeding volume of ethyl acetate is 2 times that of DMSO.
The synthesis route is:
[Main reference materials]
[1] Yuan Geng; Ren Jianwei. Preparation process of 3-bromo-5-trifluoromethylaniline. CN200610096987.7, application date 2006-10-23
[2] Wen Ming; Xu Tianhua; Wu Zhonghua; He Qilei. A nilotinib intermediate 3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl ) Preparation method of aniline.CN201610363885.0, application date 2016-05-27

 微信扫一扫打赏
微信扫一扫打赏

