[Background and Overview][1][2]
Bitter taste is one of the basic taste forms recognized by humans. Although little was known about the physiology of bitter taste in the past, and not much is currently known, it was only in 2006 that researchers at the Monell Chemical Senses Center in Philadelphia, USA, succeeded in making mature taste receptor cells cultured in vitro survive for more than a certain period. time. But the biology of taste has now begun to be elucidated, and is still in the ascendant. At present, the principle of bitter taste formation has been elucidated. The bitter taste receptor protein distributed on the microvilli on the top of the taste cells of the taste buds binds to the bitter molecules dissolved in saliva and is activated. After intracellular signal transduction, it is finally integrated by the nerve center to produce bitter taste perception.
Bitter, Benzylammonium benzoate (Denatonium Benzoate, DB) trade name is Bitrex, a bitter compound. Together with Denatonium Saccharide (DS), it is also known as the bitterest substance in the world. Bitters, discovered by a Scottish pharmaceutical company in 1958, has even been included in the Guinness Book of World Records. Usually, people can feel bitter at a concentration of only 10 ppb (10 parts per billion), and 50 ppb is more bitter. When the concentration reaches 20 to 30 ppm (parts per million), the bitterness is unbearable. Molecular formula: C28H34N2O3·H2O Molecular weight: 464.60, anhydrous 146.59. Studies have confirmed that the oral LD50 of bitters is: 485 740mg/kg in rats and 508g/kg in rabbits. Due to the hydrophilicity of quaternary ammonium salts (the solubility of bitters in water is 5%), they are difficult to pass through the blood-brain barrier and have low central nervous system toxicity. Previous studies have shown that bitters have low toxicity to fish and shrimp and that bitters are non-mutagenic. Because the concentration used is extremely small, it is generally considered safe.
[Application][2]
Bitters are currently used as aversive agents, denaturants, food inhibitors and flavoring agents. Bitters are listed as an inactive ingredient in the United States Pharmacopeia USP32 NF27 edition. However, there are no reports on the use of bitter taste receptor antagonists or inverse agonists in pharmaceutical preparation, nor in the preparation of pharmaceuticals for the treatment and/or prevention of obesity, diabetic peripheral neuropathy, and neuropathic pain. reports of use. Benzyl ammonium benzoate (bitters) is the most bitter substance known to man. Because of its low toxicity, no pollution to the environment, and no impact on other properties of the added substances, it has been used in many developed countries such as the United Kingdom, the United States, France, and Japan. Adding it to the toxic chemicals that people come into contact with in daily life and production can prevent people from accidentally ingesting it and causing poisoning.
The United Kingdom and France have published application patents for this compound as an ethanol denaturant; in recent years, the number of application patents on this compound has gradually increased. For example, Japanese patent JP 02127496 uses it as a bittering agent for alcohol-containing solid fuels, and French patent 2726832 uses it as a bittering agent for alcohol-containing solid fuels. As a bittering agent for refrigerants, etc., benzyl ammonium benzoate (bitter) is not mentioned in many patents as methanol, pesticides, fungicides, plant growth regulators, rodenticides, sodium nitrite, etc. that people come into contact with in daily life. Bitters for toxic chemicals. Some studies have conducted research on the use of benzyl ammonium amide as a bittering agent for methanol, pesticides, fungicides, plant growth regulators, rodenticides, sodium nitrite and other toxic chemicals that people come into contact with in daily life. It was found that extremely trace amounts (1~ 30ppm) benzyl ammonium benzoate can give the above chemicals a bitter taste and can effectively prevent people from accidentally ingesting them.
【Preparation】[1][3]
Method 1: Synthesis method of benzyl ammonium benzoate (bitter), using lidocaine, benzyl halide, benzoic acid, metal hydroxide as raw materials, which is characterized by using a two-pot method to synthesize benzyl ammonium benzoate amide (Bitter), the two-pot synthesis process is:
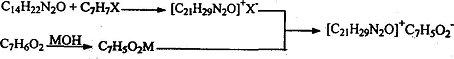
Add benzyl halide into the reactor containing lidocaine under stirring and at a certain temperature. After the reaction is complete, stop the reaction. After the obtained benzyl lidocaine ammonium halide intermediate product is purified, add the solvent , add benzoic acid under stirring. After the reaction is completed, the generated benzylammonium benzoate (bitter) solution is filtered, washed, concentrated, and washed to obtain the product benzylammonium benzoate (bitter). The reaction temperature range is : Room temperature to 130°C, of which the reaction temperature for the first step is preferably 50-105°C, and the reaction temperature for the second and third steps is preferably room temperature to 90°C. Reaction raw material ratio: the ratio of lidocaine and benzyl halide is 1:1 ~ 1:2; the ratio of lidocaine, benzyl halide and benzoate is equimolar concentration, and the reaction formula is: where, X =Cl, Br; M=Na, K.
Method 2: The synthesis method of benzyl ammonium benzoate (bitter) includes the following steps:
1) Add benzyl halide into the reactor containing raw material lidocaine under stirring and at a certain temperature. The reaction temperature is room temperature to 130°C. The ratio of lidocaine to benzyl halide is 1:1. ~1:2;
2) After the reaction is complete, add solvent and metal hydroxide;
3) Add benzoic acid. After the reaction, the generated benzyl ammonium amide (bitters) is filtered, washed, concentrated and washed to obtain the product benzyl ammonium amide (bitters).
[Main reference materials]
[1] Wang Yulan. Benzyl ammonium benzoate (picrin)��) synthesis method. CN98100785.6, application date 1998-03-24
[2] Wang Yulan. Application of benzyl ammonium benzoate as a bittering agent. CN98101236.1, application date 1998-04-01
[3] Lu Yan. Synthesis method of benzyl ammonium benzoate (bitter). CN201410635895.6, application date 2014-11-13

 微信扫一扫打赏
微信扫一扫打赏

