Background and overview【1】
P-Toluenesulfinic acid is a special type of fine intermediate and an important raw material in organic synthesis. It plays an irreplaceable role in the synthesis of certain dyes, pesticides and pharmaceutical intermediates. However, since sodium p-toluenesulfinate is catalyzed by metallic zinc in the traditional process, a large amount of waste will be produced after the reaction.
Preparation
1. Instruments and experimental reagents
Circulating water vacuum pump SHZ-D (III), vacuum drying oven DP201, magnetic stirrer DF-lO1B, LC100 Wufeng high performance liquid chromatograph, UV2450 ultraviolet spectrophotometer.
p-Toluene sulfonphthalein chloride, anhydrous sodium sulfite, sodium bicarbonate, methylene chloride, ether, acetone, chloroform.
2. Experimental process and steps
Add 0.03mol anhydrous sodium sulfite and 20mL distilled water into a 100mL four-neck flask, turn on the magnetic stirring and heating device, and connect the water separator device; after the sodium sulfite is completely dissolved, control the temperature between 4055°C and drip the liquid A solution of 0.02 mol p-toluenesulfonphthalein chloride dissolved in 10 mL methylene chloride was added dropwise into the funnel. At the same time, a saturated sodium bicarbonate solution was added dropwise to control the pH between 7 and 8. After the raw material was added dropwise, the methylene chloride was separated from the solution. Steam and recover in a water vessel; continue to raise the temperature to the reflux temperature and react for 1 hour; transfer the reaction solution to a small beaker, cool and crystallize, observe the precipitated solid, drop an appropriate amount of hydrochloric acid solution to precipitate the solid product, suction filtration, and vacuum drying to obtain the final sulfenic product. Acid products. The products were analyzed by liquid chromatography using the normalization method.
3. Results and discussion
P-Toluenesulfonphthalein chloride can easily hydrolyze with water to form sulfonic acid at a certain temperature and pH value. The competitive reaction of hydrolysis will reduce the yield of the entire reaction. Therefore, adding solvent and studying temperature and pH are extremely important in process improvement.
3.1 Effect of solvent addition
The raw material p-toluene sulfonphthalein chloride is insoluble in water, but hydrolysis reaction easily occurs in water; the reducing agent sodium sulfite is insoluble in any organic solvent and is only soluble in water. In order to reduce the hydrolysis reaction of phthalate chloride, add a low boiling point organic solvent to dissolve p-toluenesulfonphthalate chloride, then drop the solution into the aqueous solution of sodium sulfite, distill the low organic solvent, and then continue to heat up the reaction. This method replaces direct feeding. The solvent selection requirements include: (1) not reacting with phthalic chlorine; 2) having certain solubility for phthalic chlorine; 3) low boiling point, easy to evaporate and recover. The following experiments were conducted on four solvents: methylene chloride, diethyl ether, chloroform and acetone, as well as direct feeding.
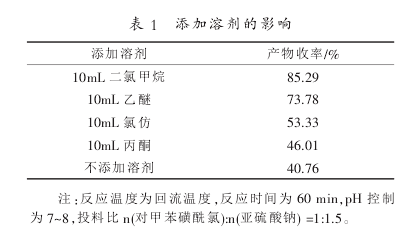
According to the analysis of the results in Table 1, the reaction effect of adding solvent is good first, and the direct addition of phthalochloride is prone to hydrolysis reaction. Secondly, from the yield of the product in different solvents, it was found that the effect was better in diethyl ether and dichloromethane. Since the boiling point of dichloromethane is 39.8°C and the boiling point of diethyl ether is 34.6°C, there is not much difference between the two from the boiling point point of view. Easily evaporated without affecting the reaction. Considering the solubility of p-toluenesulfonphthalein chloride, the solubility of ether to p-toluenesulfonphthalein chloride is not as good as methylene chloride, so dichloromethane is chosen to dissolve p-toluenesulfonphthalein chloride.
3.2 pH control
If the pH value of the sodium sulfite reaction solution is too low, it will acidify and release sulfur dioxide. If the pH value is too high, it will cause the hydrolysis of p-toluenesulfonphthalide chloride, so the appropriate pH value has a great influence on the reaction. The specific investigation results of pH are as follows:
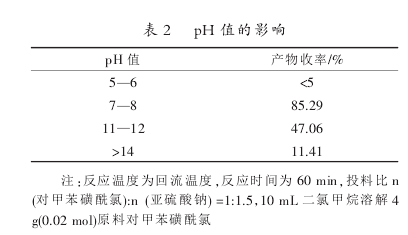
According to the results in Table 2, under acidic conditions, the reducing agent cannot exist stably after heating, and the reaction cannot proceed; in an excessively alkaline environment, phthal chloride will be hydrolyzed under heating. To sum up, it is appropriate to choose a pH value under weakly alkaline conditions and control the pH value between 7 and 8.
3.3 Effect of reaction temperature
The effect of reaction temperature on product yield was examined. The results are shown in Table 3:
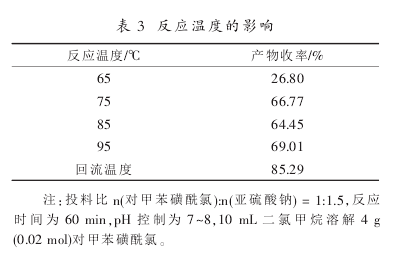
According to the analysis of the results in Table 3, the yield is basically the same when the reaction temperature is between 75°C and 95°C, and the reaction effect is best at the reflux temperature. Keeping the reaction temperature relatively stable can increase the reaction rate, reduce side reactions, and make The reaction is complete, so the optimal reaction temperature is the reflux temperature.
3.4 Effect of reaction time
For the investigation of reaction time, the following five sets of experiments were conducted
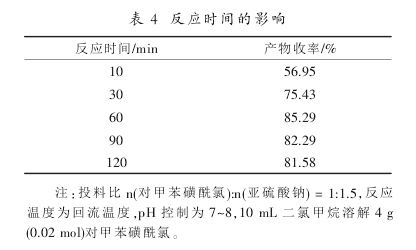
According to the analysis of the results in Table 4, the reaction has been relatively complete when the optimal reaction time is 60 minutes. If the reaction time is continued to be extended, the yield will not increase significantly, so the reaction time is 60 minutes.
The influence of 2.5 feed ratio
Sodium sulfite reduces p-toluenesulfonphthalein chloride. As the reaction proceeds, the generation of sulfinic acid reduces the pH value of the reaction system, which is not conducive to the existence of sodium sulfite. Sodium sulfite needs to be slightly excessive.
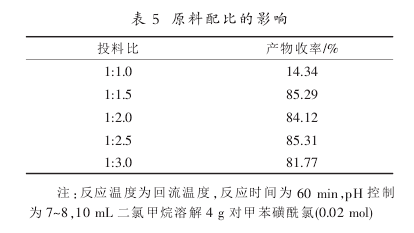
According to the analysis of the results in Table 5, when the molar ratio of p-toluenesulfonphthalein chloride and sodium sulfite is 1:1.5, the reaction yield reaches 85.29%. Continue to increase the amount of reducing agent and the reaction yield is basically the same, so it is selected. (p-Toluenesulfonphthalein chloride): n (sodium sulfite) = 1:1.5 is the optimal feeding ratio.
4. Conclusion
The optimal synthesis conditions for the reduction of p-toluenesulfonphthalate chloride by sodium sulfite aqueous solution are: dissolve the reactant sulfonphthalate chloride in methylene chloride and add it dropwise to the sodium sulfite aqueous solution. The reaction time:60 min, reaction temperature: reflux temperature, raw material feeding ratio: n (p-toluenesulfonphthalein chloride): n (sodium sulfite) = value 7~8.
5, control the pH of the reaction system
Main references
[1]Wang Kai, Xiang Bin, Shi Jiaqi. Improvement of the synthesis process of p-toluenesulfinic acid[J]. Zhejiang Chemical Industry, 2013, 44(06):34-36.

 微信扫一扫打赏
微信扫一扫打赏

