Background【1】【2】
P-Methyl sulfonyl benzaldehyde is an important pharmaceutical intermediate for florfenicol and other products. Florfenicol is a new veterinary chloramphenicol-type broad-spectrum antibacterial drug successfully developed in the late 1980s. It was first launched in Japan in 1990. In 1993, Norway approved the drug to treat salmon boils. In 1995, France, the United Kingdom, Austria, Mexico and Spain approved it for the treatment of bovine respiratory bacterial diseases. It is also approved for use as a feed additive for pigs in Japan and Mexico to prevent and treat bacterial diseases in pigs (Qiu Yinsheng et al., 1996). my country has currently approved the drug. In addition, according to research, p-methylsulfonylbenzaldehyde can also be used as an important intermediate for new human antibiotics and crop drugs. The traditional preparation of p-methyl sulfonyl benzaldehyde uses p-methyl sulfonyl toluene as the raw material and bromine as the oxidant. First, p-methyl sulfonyl dibromotoluene is obtained, and then hydrolyzed under acidic conditions to obtain the p-methyl sulfonyl benzaldehyde product. p-Methyl sulfonyl benzaldehyde is the main medium used in the production of a new generation of broad-spectrum antibiotics, thiamphenicol and its amino compounds. This achievement uses toluene as raw material and synthesizes p-methylsulfonylbenzaldehyde through processes such as chlorosulfonation, reduction, methylation, bromination, hydrolysis and alkalization. Its yield and quality have reached the domestic advanced level. According to user feedback, thiamphenicol prepared using this intermediate can significantly improve the yield and quality of the product. A production device with an annual output of 150 tons can increase output value by more than 12 million yuan and profit and tax by more than 3 million yuan. The large amount of hydrobromic acid produced by this process can be used as the basic raw material for lithium bromide refrigerant liquid, allowing the scarce chemical raw material bromine to be reused.
Introduction【3】
P-methylsulfonyl benzyl enzyme is an important raw material for the synthesis of thiamphenicol. Like chloramphenicol, thiamphenicol is a second-generation broad-spectrum antibacterial drug. They have the same antibacterial effect and mechanism of action and can inhibit Aerobic Gram-negative bacteria and Gram-positive bacteria, anaerobic bacteria, Streptococcus and Diplococcus, etc., and has better inhibitory effect on Gram-negative bacteria. However, because it is rarely combined with protein in the blood and will not be destroyed by the liver, it is higher in urine and bile than chloramphenicol, and it is a long-acting drug, so its antibacterial effect in the body is stronger than that of chloramphenicol. In addition, some Thiamphenicol has a better killing effect on bacteria that are resistant to chloramphenicol. In addition, studies have proven that thiamphenicol can also enhance the body’s immunity. A large number of studies have proven that chloramphenicol has toxic effects on bone marrow cells and liver cells of humans and animals, and can cause dose-related bone marrow suppression, or dose-independent irreversible aplastic anemia, severe gastrointestinal reactions, Superinfection, peripheral neuritis, gray baby syndrome, etc. can also cause immunosuppressive reactions and genotoxicity in broilers and mice. In addition, it is also a teratogen. In view of this, most countries in the world prohibit its clinical application and only use it as a veterinary drug. Years of clinical application have revealed that thiamphenicol also has many toxic side effects. Mainly manifested in two aspects: non-hematological toxicity and hematological toxicity. The former includes gastrointestinal reactions, abdominal pain, nausea, heartburn and vomiting, sweet spots on the skin, anorexia, acrocephaly and polyneuritis; Patients show a higher incidence of blood system disorders. In the early stage, serum iron may increase, and the number of white blood cells, hemoglobin, reticulocytes, and red blood cells may decrease slightly. In severe cases, bone marrow suppression may occur. In addition, thiamphenicol may cause peripheral neuritis. In addition, the widespread use of antibiotics in recent years has led to serious drug resistance. Therefore, it is of great practical significance to research and develop new low-toxic and efficient antibacterial drugs. In addition to being a raw material for the synthesis of thiamphenicol, p-methylsulfonyl benzoic acid itself is also an important pharmaceutical intermediate. It has a large production capacity in my country and is mainly used for the production of thiamphenicol. This article intends to utilize this cheap and easy-to-use pharmaceutical intermediate. With the raw materials obtained, chemically synthesize p-methylsulfonyl benzyl enzyme series Schiff bases, cinnamic acid and their complexes with metals, hoping to obtain leading drugs with high antibacterial activity and low toxicity.
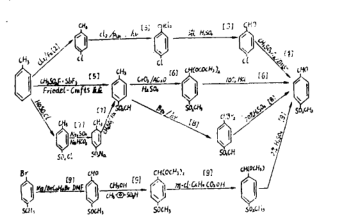
Preparation【1】
Add 161. into a 500 mL four-neck bottle in sequence. 2 g sodium methyl mercaptide, 3. 2 g phase transfer catalyst, 56. 23 g p-chlorobenzaldehyde, heated to 60℃, about 0. All dissolved in 5 hours, kept at 60-65°C for 6 hours; cooled to 20-30°C and allowed to stand for 1 hour to separate into layers, with the lower yellow oil layer 66. 67 g, water layer 153 g, intermediate yield 109. 51%, transfer the yellow organic layer to a 100 mL dropping funnel for later use. 1. 3. 2. Preparation of p-methylsulfonylbenzaldehyde product. Add 113. to a 500 mL four-neck bottle in sequence. 4 g30% hydrogen peroxide, 50 g water, 1. 6 g of concentrated sulfuric acid, 2 g of catalyst, keep the temperature at 40-45°C and slowly add the intermediate dropwise for about 1 hour. During the dropwise addition, it first becomes turbid. When the dropwise addition reaches about 1/2, a large amount of white solid precipitates. Add dropwise After completion, the temperature was raised to 60°C and kept for 5 hours. 1. 3. 3. After post-treatment, lower the temperature to 15-20℃, adjust the pH value = 9 with 10% sodium hydroxide aqueous solution, filter with suction, wash with water to pH value = 7, and dry at 85℃ to obtain 70 g of a white powdery solid product.
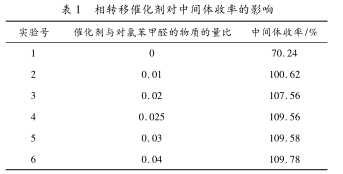
The addition of phase transfer catalyst enables the two phases immiscible with sodium methylmercaptide aqueous solution and p-chlorobenzaldehyde to react more fully. As can be seen from Table 1.��As the phase transfer catalyst is added, the yield of the intermediate gradually increases. However, when it reaches a certain level, the increase in the amount of catalyst does not promote the product yield, and causes post-processing difficulties and increased costs. The comprehensive cost considers the catalyst and The substance ratio of p-chlorobenzaldehyde is 0. 025:1 is the most suitable.
The effect of the ratio of sodium methylmercaptide and p-chlorobenzaldehyde on the intermediate:
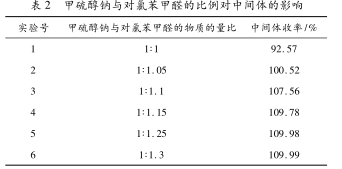
The ratio of sodium methyl mercaptide and p-chlorobenzaldehyde as the main reaction raw materials in this experiment has an extremely important impact on the reaction. 20% sodium methyl mercaptide aqueous solution is used in this raw material because this raw material is relatively cheap. , the filtration method is used to promote the reaction. It can be seen from Table 2 that when the material ratio of sodium methylmercaptide to p-chlorobenzaldehyde is 1. 15:1 is the most economical and has a higher conversion rate.
References
[1]Wu Duokun, Zhang Guoqiang, Qin Shanbao. New synthesis process of p-methylsulfonylbenzaldehyde[J]. Shandong Chemical Industry, 2016, 45(21):1-2.
[2] Editorial Department of Encyclopedia of China’s Technological Achievements, Encyclopedia of China’s Technological Achievements (Industrial Issue No. 13), Issue 18, Total Issue 98, Science and Technology Literature Press, January 1993, 1st Edition, Page 248
[3] Wang Feiwu, Hu Zhongkan. Illustration of the synthesis route of p-methylsulfone benzaldehyde [J]. Modern Applied Pharmacy, 1991(05):18.

 微信扫一扫打赏
微信扫一扫打赏

