Background and overview[1][2]
Bromoaniline is a very important chemical intermediate, which is widely used in the synthesis of fine chemical products such as medicines, dyes, and pigments. Among them, p-bromoaniline can be used as an intermediate for anticancer drugs and coumarin fluorescent dyes.
Preparation[1]
Using Raney-Ni as a catalyst, p-bromoaniline is prepared by hydrogenating and reducing p-bromonitrobenzene. Since bromine is often lost due to hydrogenolysis during the catalytic hydrogenation of brominated nitrobenzene to produce brominated aniline, a small amount of cocatalyst is added to inhibit debromination. Lu Lihua et al. reported the use of W-4 type Raney-Ni as the catalyst, methanol as the solvent, and dicyandiamide as the cocatalyst to catalytically hydrogenate and reduce p-bromonitrobenzene to prepare p-bromoaniline, and the conversion rate of p-bromonitrobenzene. It can reach more than 99.8%. The molar selectivity of p-bromoaniline reaches 98.0%, and the catalyst can be recycled. The process is simple and easy to operate. The solvent methanol can also be recycled. There is basically no environmental pollution and it is suitable for industrial production.
Apply[2]
1. Preparation of polyaniline/n‑ type single crystal silicon composite electrode material
Among the many functional organic molecules, conjugated conductive polymers are widely used in organic optoelectronic devices and electronics due to their special electrical and optical properties, flexible mechanical properties, processability, and electrochemical redox activity. It has played an important role in the development and development of chemical devices, attracting more and more attention and research at home and abroad. Among them, representative conjugated conductive polymers include polyacetylene, polypyrrole, polyaniline, polythiophene, polyparaphenylenevinylene, polyparaphenylene, etc. Among many types, polyaniline has received increasing attention due to its superior electro-optical properties. However, there are few reports on the effect of polyaniline on the photocurrent effect after being assembled on the surface of single crystal silicon.
CN201110085742.5 synthesized polyaniline/n-type composite electrode material, and found that the obtained electrode material has good photoelectric conversion effect. Polyaniline/n-type single crystal silicon composite material is a sulfonated polyaniline system that is connected to the single crystal silicon surface through the C atoms on the benzene ring and the Si atoms on the single crystal silicon surface in the form of Si-C covalent bonds. Under simulated sunlight, the photocurrent density value of this composite material can reach 3.446mA·cm‑2, and the open circuit voltage can reach ‑0.300 V. The preparation method is: first use p-bromoaniline to graft onto the surface of single crystal silicon, then use this as the base material to prepare polyaniline/n-type composite electrode through polymerization reaction, and link sulfonate groups on the benzene ring of the polymerized organic chain, finally obtaining Sulfonic acid modified polyaniline/n-type single crystal silicon composite. The reaction mechanism is as follows:
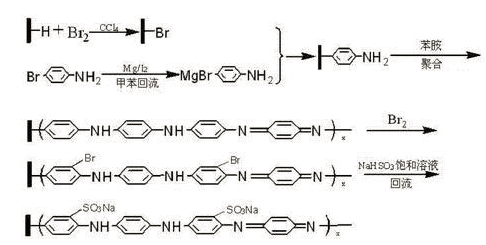
The specific preparation steps are as follows:
A. First clean the surface of the n‑ type single crystal silicon wafer, then immerse it in 5% hydrogen fluoride and 40% ammonium fluoride solution, and then use deionized water Rinse, vacuum dry, and store in a nitrogen atmosphere to obtain a surface hydrogenated single crystal silicon wafer; for the surface cleaning method of the n- type single crystal silicon wafer, please refer to the document J.Applied Physics Letters 56 (1990) 656- 658.
B. Immerse the surface hydrogenated silicon wafer into a carbon tetrachloride solution with a liquid bromine concentration of 3-6g·L-1 for 10-15 minutes, take it out and use tetrachloride solution Rinse the carbon, dry it in a vacuum, and store it in a nitrogen atmosphere to obtain a surface brominated single crystal silicon wafer; use toluene as the solvent to prepare the corresponding Grignard reagent for p-bromoaniline—anilino magnesium bromide (MgBr-C6H5, its concentration 5.5-6.2mol·L–1), mix this formatting reagent with the surface brominated single crystal silicon wafer under nitrogen protection, and heat and reflux for 10-15 minutes Afterwards, the silicon wafer was taken out, washed several times with acetone and methylene chloride, vacuum dried, and stored in a nitrogen atmosphere to obtain a single crystal silicon wafer with an aniline-modified surface. For the preparation of Format reagent—anilino magnesium bromide, please refer to the literature J. Hebei Chemical Industry 30 (2007) 27-28.
C. Prepare a mixed solution of aniline and hydrochloric acid, in which the concentration of aniline is 0.20‑0.22mol·L‑1 and the concentration of hydrochloric acid is 1.0‑1.1 mol·L‑1, immerse the single crystal silicon wafer with an aniline-modified surface into this mixed solution with a volume of 10‑15mL, and add 0.20‑0.22mol·L‑1 ammonium peroxodisulfate aqueous solution 10‑dropwise 15mL and stir vigorously, react at 0~5℃ for 4-5 hours, then take out the silicon wafer and rinse it with 0.50-0.55mol·L-1 hydrochloric acid solution; immerse the above silicon wafer in 0.10-0.12mol ·L‑1 sodium hydroxide solution for 2‑2.5 hours, take it out and rinse with a large amount of deionized water; then immerse the above silicon wafer in N‑methylpyrrolidone for 48‑50 hours, rinse with a large amount of ethanol Rinse with deionized water and dry under vacuum; immerse the dried silicon wafer in 1.0-1.2mol·L-1 hydrochloric acid solution for 10-12 hours, then take it out and rinse it with deionized water several times , dried in vacuum, and stored in a nitrogen atmosphere, a single crystal silicon wafer with a polyaniline-modified surface was obtained.
D. Immerse the polyaniline-modified single crystal silicon wafer on the surface into 10-20mL liquid bromine and let it stand for 10-14 days. Then take out the silicon wafer and rinse it with carbon tetrachloride; then immerse it in 25-30mL saturated The polyaniline/n-type single crystal silicon composite material is obtained by heating and refluxing in a sodium bisulfite solution for 10-12 hours.
2. Preparation of 3D printer base material based on cattail grass modification
A method for preparing a 3D printer base material based on cattail grass modification, including:
1) Kill and crush the cattail powder, then mix the cattail powder and bamboo charcoal powder and soak them in a mixed solution of p-bromoaniline and hydrochloric acid, and then filter the filter cake to prepare the modified composition;
2) Combine expanded polystyrene, modified composition, polyethylene, polyimide, white carbon black, 2-thiol phenylthiazole, dilauryl thiodipropionate, steel fiber, Mica powder, dioctyl subebate and aluminum acetylacetonate are mixed, then melted, cooled and granulated to make a 3D printer base material.
Main reference materials
[1] Lu Lihua, Li Yingchun. Preparation of p-bromoaniline by catalytic hydrogenation[J]. Journal of Qingdao University of Science and Technology (Natural Science Edition), 2005(03):205-207.
[2]CN201110085742.5 Polyaniline/n-type single crystal silicon composite electrode material and preparation method thereof
[3]CN201510975571.1 3D printer base material based on cattail grass modification and its preparation method

 微信扫一扫打赏
微信扫一扫打赏

