Synthesis【1】
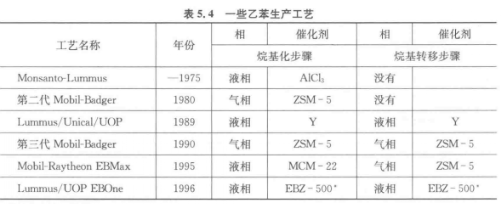
The alkylation process of benzene and ethylene was developed in the 1930s. It is carried out in the liquid phase in the presence of a Friedel-Crafts catalyst such as A1C13-HCl. Since there are many inconveniences when using liquid acid, acid-supported catalysts have been proposed. In the 1960s, BF3/AI2O3 and solid phosphoric acid (SPA, diatomaceous earth loaded phosphoric acid) began to be used. These catalysts release acid during the reaction and cannot be regenerated after the reaction. So attempts were made to use SIO2– AI2O3 and faujasites such as HY and HX, but these catalysts were seriously deactivated. Therefore, A1C13-HCl was used in industry until the 1980s.
In the 1980s and 1990s, some industrial alkylation processes using zeolites came into use. The first industrial process using zeolites began in the 1980s, using medium-pore zeolite ZSM-5, and the reaction was carried out in a gas-phase fixed-bed reactor (second-generation Mobil-Badger process). Using Y zeolite with large pores in the liquid phase, the deactivation is not serious. The process using Y zeolite was commercialized in 1989 (Lummus/Unocal/UOP). the year 1995. A new process using MCM-22 (Mobil-Raytheon EBMax) has emerged.
Ethylbenzene is obtained by acid-catalyzed phenethylation. The generated ethylbenzene is further alkylated under the action of acid to become disubstituted and other multi-substituted ethylbenzenes. Multi-substituted ethylbenzene is separated from ethylbenzene and then transalkylated with benzene to increase the yield of ethylbenzene. The transalkylation reaction is also an acid-catalyzed reaction, but the catalyst is different from that of alkylation. Therefore, the alkylation process often consists of two steps: alkylation and transalkylation.
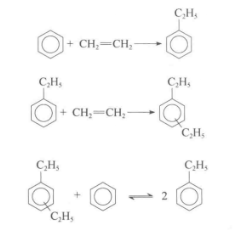
The catalysts currently used in the alkylation process include HY, ZSM-5, MCM-22 and beta-zeolite. Since the molecular size of polysubstituted ethylbenzene is larger than that of ethylbenzene, the catalyst used in the transalkylation process has slightly larger pores than the alkylation catalyst. The choice of appropriate pore size depends on whether the reaction is a gas phase or a liquid phase reaction.
The following table lists some ethylbenzene production processes.
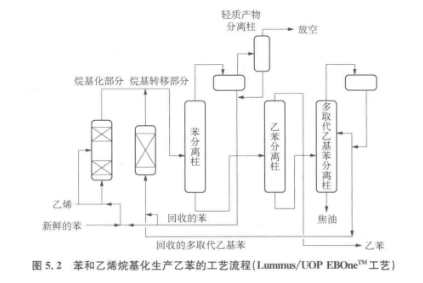
The figure below is a schematic flow diagram of the alkylation process.
Absorption, metabolism and excretion【2】
This product can be absorbed through the respiratory tract, skin and digestive tract. It is more easily absorbed through the skin and can even cause death. However, no poisoning was seen under production conditions. At a concentration of 99 to 369 mg/m3, the absorption rate through the human respiratory tract is approximately 64% within 8 hours. Most of it is oxidized (via phenylethyl alcohol) into benzoic acid (25%) and mandelic acid (64%) in the body, and is quickly excreted from the urine (more than 80% is excreted in 24 hours). A small amount (less than 10%) is excreted in its original form through the body. Excreted by lungs and kidneys. Very little is excreted in the form of phenylacetic acid/phenylethyl alcohol and benzoic acid. Therefore, benzoic acid and mandelic acid in urine can be measured as indicators of exposure to ethylbenzene.
Dangerous Characteristics
The vapor and air form an explosive mixture, which can cause combustion and explosion when exposed to open flames or high heat. It can react with oxidants. Its vapor is heavier than air and can spread to a considerable distance from a lower place. It will cause backfire when exposed to an open flame. If exposed to high heat, the internal pressure of the container will increase and there is a risk of cracking and explosion. If the flow rate is too fast, it is easy to generate and accumulate static electricity. Its vapor and smoke are irritating to eyes, mucous membranes, respiratory tract and skin. Inhalation or accidental ingestion may cause headache, nausea, vomiting and decreased function of the central nervous system. Direct inhalation of this product’s liquid can cause pulmonary edema, bleeding and chemical pneumonitis. Suspected human carcinogen.
Storage and transportation precautions【3】
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The warehouse temperature should not exceed 30℃. Protect from direct sunlight. Keep container tightly sealed. Should be stored separately from oxidizing agents. Lighting, ventilation and other facilities in storage should be explosion-proof. Equipped with the appropriate variety and quantity of fire equipment. Fire and explosion prevention technical measures must be taken during tank storage. It is prohibited to use mechanical equipment and tools that are prone to sparks. When filling, pay attention to the flow rate (not exceeding 3m/s), and have a grounding device to prevent the accumulation of static electricity. During transportation, load and unload with care to prevent damage to packaging and containers.
First aid
Skin contact: Take off contaminated clothing and wash thoroughly with soap and water. Seek medical attention.
Eye contact: Open the upper and lower eyelids immediately and rinse with running water or saline for at least 15 minutes. Seek medical attention.
Inhalation: Leave the scene quickly to fresh air. Keep your airway open. Keep warm and rest. Give oxygen if breathing is difficult. Once breathing stops, begin CPR immediately. Seek medical attention.
Ingestion: If swallowed by mistake, rinse your mouth immediately, drink enough warm water, and perform gastric lavage as soon as possible. Seek medical attention.
Protective measures
Engineering control: The production process is sealed and ventilation is enhanced.
Respiratory system protection: Wear a gas mask when the concentration in the air exceeds the standard. During emergency rescue or evacuation, it is recommended to wear self-contained breathing apparatus.
Eye protection: Wear chemical safety glasses when exposed to high concentration vapor.
Protective clothing: Wear anti-static clothing.
Hand protection: Wear chemical-resistant gloves. Skin shields may also be used.
Others: Smoking, eating and drinking are prohibited at the work site. After work, shower and change clothes. Maintain good hygiene habits.
Leakage disposal
Evacuate personnel in the leaked contaminated area to a safe area, prohibit irrelevant personnel from entering the contaminated area, and cut off the source of fire. Emergency responders wear self-contained breathing apparatus and general fire protective clothing. Plug the leak while ensuring safety. Spray water mist to reduce evaporation. Absorb with activated carbon or other inert materials. Then use non-sparking tools to collect and transport it to a waste disposal site. You can also use emulsion made of non-flammable dispersants for scrubbing, and the diluted lotion can be put into the wastewater system. If there is a large amount of leakage, use dikes to contain it, then collect, transfer, recycle or dispose of it after harmless treatment.
References
[1] (Japanese) Hideshi Hattori, Yoshio Ono; Translated by Gao Zi, Le Yinghong, Hua Weiming, Solid acid catalysis, Fudan University Press, 2016.05, page 202
[2] Edited by the writing team of “Industrial Toxicology”, Industrial Toxicology Volume 2, Shanghai People’s Publishing House, August 1977, 1st edition, page 433
[3] Editor-in-chief Wang Guangsheng; deputy editor-in-chief Zhang Haifeng, Petrochemical Raw Materials and Product Safety Manual, Sinopec Press, 2010.08, page 30

 微信扫一扫打赏
微信扫一扫打赏

